Pulmonary Complications of Cystic Fibrosis
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Rhinotillexomania in a Cystic Fibrosis Patient Resulting in Septal Perforation Mark Gelpi1*, Emily N Ahadizadeh1,2, Brian D’Anzaa1 and Kenneth Rodriguez1
ISSN: 2572-4193 Gelpi et al. J Otolaryngol Rhinol 2018, 4:036 DOI: 10.23937/2572-4193.1510036 Volume 4 | Issue 1 Journal of Open Access Otolaryngology and Rhinology CASE REPORT Rhinotillexomania in a Cystic Fibrosis Patient Resulting in Septal Perforation Mark Gelpi1*, Emily N Ahadizadeh1,2, Brian D’Anzaa1 and Kenneth Rodriguez1 1 Check for University Hospitals Cleveland Medical Center, USA updates 2Case Western Reserve University School of Medicine, USA *Corresponding author: Mark Gelpi, MD, University Hospitals Cleveland Medical Center, 11100 Euclid Avenue, Cleveland, OH 44106, USA, Tel: (216)-844-8433, Fax: (216)-201-4479, E-mail: [email protected] paranasal sinuses [1,4]. Nasal symptoms in CF patients Abstract occur early, manifesting between 5-14 years of age, and Cystic fibrosis (CF) is a multisystem disease that can have represent a life-long problem in this population [5]. Pa- significant sinonasal manifestations. Viscous secretions are one of several factors in CF that result in chronic sinona- tients with CF can develop thick nasal secretions con- sal pathology, such as sinusitis, polyposis, congestion, and tributing to chronic rhinosinusitis (CRS), nasal conges- obstructive crusting. Persistent discomfort and nasal man- tion, nasal polyposis, headaches, and hyposmia [6-8]. ifestations of this disease significantly affect quality of life. Sinonasal symptoms of CF are managed medically with Digital manipulation and removal of crusting by the patient in an attempt to alleviate the discomfort can have unfore- topical agents and antibiotics, however surgery can be seen damaging consequences. We present one such case warranted due to the chronic and refractory nature of and investigate other cases of septal damage secondary to the symptoms, with 20-25% of CF patients undergoing digital trauma, as well as discuss the importance of sinona- sinus surgery in their lifetime [8]. -

Cryptogenic Organizing Pneumonia—Results of Treatment with Clarithromycin Versus Corticosteroids—Observational Study
RESEARCH ARTICLE Cryptogenic organizing pneumoniaÐResults of treatment with clarithromycin versus corticosteroidsÐObservational study Elżbieta Radzikowska1*, Elżbieta Wiatr1☯, Renata Langfort2³, Iwona Bestry3³, Agnieszka Skoczylas4, Ewa Szczepulska-Wo jcik2³, Dariusz Gawryluk1☯, Piotr Rudziński5³, Joanna Chorostowska-Wynimko6³, Kazimierz Roszkowski-Śliż1³ 1 III Department of Lung Disease National Tuberculosis and Lung Diseases Research Institute, Warsaw, Poland, 2 Pathology Department National Tuberculosis and Lung Diseases Research Institute, Warsaw, Poland, 3 Radiology Department National Tuberculosis and Lung Diseases Research Institute, Warsaw, a1111111111 Poland, 4 Geriatrics Department National Institute of Geriatrics, Rheumatology and Rehabilitation, Warsaw, a1111111111 Poland, 5 Thoracic Surgery Department National Tuberculosis and Lung Diseases Research Institute, a1111111111 Warsaw, Poland, 6 Laboratory of Molecular Diagnostics and Immunology National Tuberculosis and Lung Diseases Research Institute, Warsaw, Poland a1111111111 a1111111111 ☯ These authors contributed equally to this work. ³ These authors also contributed equally to this work. * [email protected] OPEN ACCESS Abstract Citation: Radzikowska E, Wiatr E, Langfort R, Bestry I, Skoczylas A, Szczepulska-WoÂjcik E, et al. (2017) Cryptogenic organizing pneumoniaÐ Background Results of treatment with clarithromycin versus Cryptogenic organizing pneumonia (COP) is a clinicopathological syndrome of unknown ori- corticosteroidsÐObservational study. PLoS ONE 12(9): e0184739. -

Does Cystic Fibrosis Constitute an Advantage in COVID-19 Infection? Valentino Bezzerri, Francesca Lucca, Sonia Volpi and Marco Cipolli*
Bezzerri et al. Italian Journal of Pediatrics (2020) 46:143 https://doi.org/10.1186/s13052-020-00909-1 LETTER TO THE EDITOR Open Access Does cystic fibrosis constitute an advantage in COVID-19 infection? Valentino Bezzerri, Francesca Lucca, Sonia Volpi and Marco Cipolli* Abstract The Veneto region is one of the most affected Italian regions by COVID-19. Chronic lung diseases, such as chronic obstructive pulmonary disease (COPD), may constitute a risk factor in COVID-19. Moreover, respiratory viruses were generally associated with severe pulmonary impairment in cystic fibrosis (CF). We would have therefore expected numerous cases of severe COVID-19 among the CF population. Surprisingly, we found that CF patients were significantly protected against infection by SARS-CoV-2. We discussed this aspect formulating some reasonable theories. Keywords: Cystic fibrosis, SARS-CoV-2, Covid-19, Azythromycin, DNase Introduction status, one would surmise that CF patients would be at The comorbidities of obesity, hypertension, diabetes, an increased risk of developing severe COVID-19 illness. heart failure, and chronic lung disease have been associ- ated with poor outcome in coronavirus disease 2019 Methods (COVID-19) [1]. Once Severe Acute Respiratory Syn- We conducted a retrospective study of 532 CF patients – drome (SARS) Coronavirus (CoV)-2 has infected host followed at the Cystic Fibrosis Center of Verona, Italy. cells, excessive inflammatory and thrombotic processes SARS-CoV-2 positivity was tested by collecting com- take place. A cytokine storm release with markedly ele- bined nose-throat swabs and subsequent Real-Time PCR vated IL-6 levels are associated with increased lethality using the Nimbus MuDT tm (Seegene, Seoul, South [2]. -

Bronchodilator Responsiveness in Children with Cystic Fibrosis and Allergic Bronchopulmonary Aspergillosis
AGORA | RESEARCH LETTER Bronchodilator responsiveness in children with cystic fibrosis and allergic bronchopulmonary aspergillosis Mordechai Pollak 1, Michelle Shaw2, David Wilson1, Hartmut Grasemann1,2 and Felix Ratjen1,2 Affiliations: 1Division of Respiratory Medicine, Hospital for Sick Children, Toronto, ON, Canada. 2Translational Medicine, Sickkids Research Institute, Toronto, ON, Canada. Correspondence: Mordechai Pollak, Hospital for Sick Children, SickKids Learning Institute, Respiratory Medicine, 555 University Ave, Toronto, ON M5G 1X8, Canada. E-mail: [email protected] @ERSpublications CF patients with a new diagnosis of ABPA had a similar BD response, compared to CF patients with acute lung function deterioration from other causes. BD response testing did not help differentiating ABPA from other causes of lung function deterioration. https://bit.ly/39Oegnh Cite this article as: Pollak M, Shaw M, Wilson D, et al. Bronchodilator responsiveness in children with cystic fibrosis and allergic bronchopulmonary aspergillosis. Eur Respir J 2020; 56: 2000175 [https://doi.org/ 10.1183/13993003.00175-2020]. This single-page version can be shared freely online. To the Editor: Allergic bronchopulmonary aspergillosis (ABPA) is a hypersensitivity lung disease that occurs in approximately 9% of children with cystic fibrosis (CF) [1]. While ABPA is commonly associated with worsening lung function, differentiating ABPA from other causes of pulmonary function decline often poses a clinical challenge. This is reflected by major differences among the various diagnostic criteria for ABPA that have been suggested to date [2–5]. A positive bronchodilator response (BDR) is characteristic for asthma which is a common co-morbidity in CF patients, but whether this is helpful in differentiating ABPA from other causes of deterioration in lung function is currently unclear. -

Infection Control Recommendations for Patients with Cystic Fibrosis
S6 INFECTION CONTROL AND HOSPITAL EPIDEMIOLOGY May 2003 INFECTION CONTROL RECOMMENDATIONS FOR PATIENTS WITH CYSTIC FIBROSIS: MICROBIOLOGY, IMPORTANT PATHOGENS, AND INFECTION CONTROL PRACTICES TO PREVENT PATIENT-TO-PATIENT TRANSMISSION Lisa Saiman, MD, MPH; Jane Siegel, MD; and the Cystic Fibrosis Foundation Consensus Conference on Infection Control Participants EXECUTIVE SUMMARY (d) The previously published HICPAC/CDC guidelines for Infection Control Recommendations for Patients With prevention of healthcare-associated infections have not Cystic Fibrosis: Microbiology, Important Pathogens, and included background information and recommenda- Infection Control Practices to Prevent Patient-to-Patient tions for the specific circumstances of patients with CF. Transmission updates, expands, and replaces the con- Thus, specific guidelines for CF patients are needed. sensus statement, Microbiology and Infectious Disease in (e) The link between acquisition of pathogens and morbidity Cystic Fibrosis published in 1994.1 This consensus docu- and mortality is well established. Prevention of acquisi- ment presents background data and evidence-based rec- tion of specific pathogens may further improve the mean ommendations for practices that are intended to decrease survival of CF patients, which has increased to 33.4 years the risk of transmission of respiratory pathogens among in 2001.3-9 CF patients from contaminated respiratory therapy equip- A multidisciplinary committee consisting of health- ment or the contaminated environment and thereby reduce care professionals from the United States, Canada, and the burden of respiratory illness. Included are recommen- Europe with experience in CF care and healthcare epi- dations applicable in the acute care hospital, ambulatory, demiology/infection control reviewed the relevant litera- home care, and selected non-healthcare settings. -
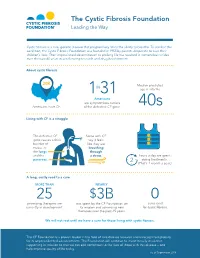
The Cystic Fibrosis Foundation Leading the Way
The Cystic Fibrosis Foundation Leading the Way Cystic fibrosis is a rare, genetic disease that progressively limits the ability to breathe. To combat this condition, the Cystic Fibrosis Foundation was founded in 1955 by parents desperate to save their children’s lives. Their impassioned determination to prolong life has resulted in tremendous strides over the past 60 years in accelerating research and drug development. About cystic fibrosis Median predicted age is into the Americans are symptomless carriers Americans have CF. of the defective CF gene. Living with CF is a struggle The defective CF Some with CF gene causes a thick say it feels buildup of like they are mucus in breathing the lungs through and the a straw. hours a day are spent pancreas. doing treatments. (That’s 1 month a year.) A long, costly road to a cure promising therapies are was spent by the CF Foundation on cures exist currently in development. its mission and advancing new for cystic fibrosis. therapies over the past 25 years. We will not rest until we have a cure for those living with cystic fibrosis. The CF Foundation is a proven leader in the field of rare disease research and is recognized globally for its unprecedented advancements. The Foundation will continue to invest heavily in science supporting its mission so that we can add tomorrows to the lives of those with this disease – and help improve quality of life today. As of September 2018 Jordan, age 22 While people with CF are living longer than in the past, we still lose precious young lives every day. -

Article First Wave of COVID-19 in French Patients with Cystic Fibrosis
Supplementary material First Wave of COVID-19 in French Patients with Cystic Fibrosis Harriet Corvol 1,2,*, Sandra de Miranda 3, Lydie Lemonnier 4, Astrid Kemgang 2, Martine Reynaud Gaubert 5,6, Raphael Chiron 7, Marie-Laure Dalphin 8, Isabelle Durieu 9, Jean-Christophe Dubus 10, Véronique Houdouin 11, Anne Prevotat 12, Sophie Ramel 13, Marine Revillion 14, Laurence Weiss 15, Loic Guillot 2, Pierre-Yves Boelle 16 and Pierre-Régis Burgel 17,18 on behalf the French Cystic Fibrosis Reference Network study group Supplementay material Expected Number and Age Distribution of COVID-19 in French Patients with Cystic Fibrosis We obtained the cumulated number of hospitalized COVID-19 cases in the French general population up to 30 June 2020. These data are open source, available from data.gouv.fr (https://www.data.gouv.fr/fr/datasets/r/41b9bd2a-b5b6-4271-8878-e45a8902ef00). Then, we obtained the probability of hospitalization upon SARS-Cov-2 infection by age from Table S1 in Salje et al. [21]. The numbers of people in each age class in France were obtained from census data (https://www.insee.fr/fr/statistiques/1892086?sommaire=1912926). From these data, we computed the extrapolated percentage of infections by age class up to 30 June 2020 in the general French population using proportionality rules. Finally, we computed the expected number of infections that would have been expected in the French CF population had the risk of infection been the same as in the general population. We used the number of CF patients from the national registry as the denominator to compute the expected number of SARS-Cov-2 CF cases [1]. -

Clinical Guidance on COVID- 19 Vaccines for People with Cystic Fibrosis
Clinical Guidance on COVID- 19 Vaccines for People with Cystic Fibrosis This guidance is intended for health-care providers. It is based on known evidence as of June 16, 2020. Background and Context The SARS-CoV-2 pandemic has been of particular concern for the cystic fibrosis (CF) community. CF is a multisystem condition with comorbidities that are expected to increase vulnerability to COVID-19. This guidance is based on a review of three of the vaccines approved by Health Canada for the prevention of COVID-19 disease caused by the SARS-CoV-2 virus: Pfizer-BioNTech (BNT162b2)1 and Moderna (mRNA-1273)2, both of which are mRNA vaccines, as well as AstraZeneca/COVISHIELD (ChADOx1-S)3 which is a replication defective adenoviral vector (‘viral vector’) vaccine. Currently, anyone aged 12+ (born in 2009 and later) in British Columbia is eligible for COVID-19 immunization. At this time, only the Pfizer-BioNTech mRNA vaccine is authorized for youth aged 12 and above,3 and we are expecting that Health Canada will authorize the Moderna mRNA vaccine for 12-17 year olds in the near future. Studies of the COVID-19 vaccines in younger children are ongoing. As per the National Advisory Committee on Immunization (NACI)4, the two mRNA vaccines authorized in Canada (Pfizer- BioNTech and Moderna) can be interchanged for the second dose to complete the series, if the vaccine received for the first dose is not available or is unknown. No data currently exist on the interchangeability of the COVID-19 mRNA vaccines. However, there is no reason to believe that mRNA vaccine series completion with a different authorized mRNA vaccine product will result in any additional safety issues of deficiency in protection. -

COVID-19 & Cystic Fibrosis
COVID-19 & Cystic Fibrosis People with cystic fibrosis (CF) may have anxiety about their health during the COVID-19 pandemic. However, there isn’t any evidence to suggest that those with CF are more likely to contract COVID-19 according to Dr. Jennifer Taylor-Cousar, co-director of the Adult Cystic Fibrosis Program at National Jewish Health. “What we do know is that those with CF are more at risk to have severe effects of COVID-19 on their lungs.” Cystic fibrosis patients are used to social distancing and taking extra precautions to keep from getting sick. In fact, these habits may be the reason that fewer than expected cystic fibrosis patients have reported contracting COVID-19. Dr. Taylor-Cousar says, “Overall, people with CF aren’t catching COVID-19 frequently, most likely because they are used to washing their hands frequently, staying home and staying six feet away from other people.” Routine Care for Cystic Fibrosis during COVID-19 The COVID-19 pandemic has made getting treatment for cystic fibrosis at health care centers harder. The Cystic Fibrosis Foundation recommends that patients see their doctors quarterly for an in depth checkup. During the pandemic, according to Dr. Taylor-Cousar, many health care providers are using telehealth to connect with CF patients. “Telehealth let’s doctors give patients advice about their medications, and anything that needs to change because of mild COVID symptoms without the patient having to leave their house,” she explained. COVID-19 Treatment for CF Patients If a cystic fibrosis patient does become sick with COVID-19, the patient’s treatment may look the same as a patient without CF. -

Allergic Bronchopulmonary Aspergillosis in a Patient with Chronic Obstructive Pulmonary Disease
Prim Care Respir J 2012; 21(1): 111-114 CASE-BASED LEARNING Allergic bronchopulmonary aspergillosis in a patient with chronic obstructive pulmonary disease Elias Mira,*Ashok Shaha a Department of Respiratory Medicine, Vallabhbhai Patel Chest Institute, University of Delhi, Delhi, India Originally received 13th April 2011; resubmitted 27th July 2011; revised 16th September 2011; accepted 17th September 2011; online 5th January 2012 Summary Allergic bronchopulmonary aspergillosis (ABPA) is a debilitating lung disease which occurs as a result of interplay between a variety of host and environmental factors. It occurs in certain susceptible individuals who develop hypersensensitivity to the colonised Aspergillus species. ABPA is a complicating factor in 2% of patients with asthma and is also seen in patients with cystic fibrosis. Asthma and chronic obstructive pulmonary disease (COPD) are known to share key elements of pathogenesis. It is well known that ABPA can occur in patients with asthma, but it has recently been reported in patients with COPD as well. We report a 55-year-old male ex-smoker who presented with complaints of exertional breathlessness and productive cough for five years and an episode of haemoptysis four days prior to presentation. Spirometery showed airflow obstruction which was not reversible with bronchodilators. Chest CT scan revealed paraseptal emphysema along with central bronchiectasis (CB) in the right upper lobe and bilateral lower lobes. A type I skin hypersensitivity reaction to Aspergillus species was elicited. He fulfilled the serological criteria for ABPA and was diagnosed as having concomitant COPD and ABPA-CB. The patient was initiated on therapy for COPD along with oral corticosteroids, on which he had remarkable symptomatic improvement. -

Cryptogenic Organizing Pneumonia
462 Cryptogenic Organizing Pneumonia Vincent Cottin, M.D., Ph.D. 1 Jean-François Cordier, M.D. 1 1 Hospices Civils de Lyon, Louis Pradel Hospital, National Reference Address for correspondence and reprint requests Vincent Cottin, Centre for Rare Pulmonary Diseases, Competence Centre for M.D., Ph.D., Hôpital Louis Pradel, 28 avenue Doyen Lépine, F-69677 Pulmonary Hypertension, Department of Respiratory Medicine, Lyon Cedex, France (e-mail: [email protected]). University Claude Bernard Lyon I, University of Lyon, Lyon, France Semin Respir Crit Care Med 2012;33:462–475. Abstract Organizing pneumonia (OP) is a pathological pattern defined by the characteristic presence of buds of granulation tissue within the lumen of distal pulmonary airspaces consisting of fibroblasts and myofibroblasts intermixed with loose connective matrix. This pattern is the hallmark of a clinical pathological entity, namely cryptogenic organizing pneumonia (COP) when no cause or etiologic context is found. The process of intraalveolar organization results from a sequence of alveolar injury, alveolar deposition of fibrin, and colonization of fibrin with proliferating fibroblasts. A tremen- dous challenge for research is represented by the analysis of features that differentiate the reversible process of OP from that of fibroblastic foci driving irreversible fibrosis in usual interstitial pneumonia because they may determine the different outcomes of COP and idiopathic pulmonary fibrosis (IPF), respectively. Three main imaging patterns of COP have been described: (1) multiple patchy alveolar opacities (typical pattern), (2) solitary focal nodule or mass (focal pattern), and (3) diffuse infiltrative opacities, although several other uncommon patterns have been reported, especially the reversed halo sign (atoll sign). -

Cystic Fibrosis
cf_new3.qxd 2/21/96 3:14 PM Page 1 FACTS ABOUT Cystic Fibrosis What Is Cystic Fibrosis What Are the Signs and Symptoms Cystic fibrosis (CF) is a chronic, progressive, of CF? and frequently fatal genetic (inherited) dis CF does not follow the same pattern in all ease of the body’s mucus glands. CF pri patients but affects different people in dif marily affects the respiratory and digestive ferent ways and to varying degrees. systems in children and young adults. The However, the basic problem is the same— sweat glands and the reproductive system an abnormality in the glands, which pro are also usually involved. On the average, duce or secrete sweat and mucus. Sweat individuals with CF have a lifespan of cools the body; mucus lubricates the respi approximately 30 years. ratory, digestive, and reproductive systems, and prevents tissues from drying out, pro CF-like disease has been known for over tecting them from infection. two centuries. The name, cystic fibrosis of the pancreas, was first applied to the disease People with CF lose excessive amounts of in 1938. salt when they sweat. This can upset the balance of minerals in the blood, which may How Common Is CF? cause abnormal heart rhythms. Going into shock is also a risk. According to the data collected by the Cystic Fibrosis Foundation, there are about Mucus in CF patients is very thick and 30,000 Americans, 3,000 Canadians, and accumulates in the intestines and lungs. 20,000 Europeans with CF. The disease The result is malnutrition, poor growth, occurs mostly in whites whose ancestors frequent respiratory infections, breathing came from northern Europe, although it difficulties, and eventually permanent lung affects all races and ethnic groups.