Water Quality, Cyanobacteria, and Environmental Factors
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Ohiocontrolled Hunting
CONTROLLED HUNTING OHIO OPPORTUNITIES 2020-2021 Application period JULY 1, 2020 to JULY 31, 2020 OHIO DEPARTMENT OF NATURAL RESOURCES DIVISION OF WILDLIFE wildohio.gov OHIO DEPARTMENT OF NATURAL RESOURCES DIVISION OF WILDLIFE The Division of Wildlife’s mission is to conserve and improve fish and wildlife resources and their habitats for sustainable use and appreciation by all. VISIT US ON THE WEB WILDOHIO.GOV FOR GENERAL INFORMATION 1-800-WILDLIFE (1-800-945-3543) TO REPORT WILDLIFE VIOLATIONS 1-800-POACHER (1-800-762-2437) DIVISION OF WILDLIFE **AVAILABLE 24 HOURS** DISTRICT OFFICES OHIO GAME CHECK OHIOGAMECHECK.COM WILDLIFE DISTRICT ONE 1500 Dublin Road 1-877-TAG-IT-OH Columbus, OH 43215 (1-877-824-4864) (614) 644‑3925 WILDLIFE DISTRICT TWO HIP CERTIFICATION 952 Lima Avenue 1-877-HIP-OHIO Findlay, OH 45840 (1-877-447-6446) (419) 424‑5000 WILDLIFE DISTRICT THREE FOLLOW US ON SOCIAL MEDIA 912 Portage Lakes Drive Akron, OH 44319 Like us on Facebook (330) 644‑2293 facebook.com/ohiodivisionofwildlife Follow us on Twitter WILDLIFE DISTRICT FOUR twitter.com/OhioDivWildlife 360 E. State Street Athens, OH 45701 (740) 589‑9930 WILDLIFE DISTRICT FIVE 1076 Old Springfield Pike Xenia, OH 45385 (937) 372‑9261 EQUAL OPPORTUNITY The Ohio Division of Wildlife offers equal opportunity regardless GOVERNOR, STATE OF OHIO of race, color, national origin, age, disability or sex (in education programs). If you believe you have been discriminated against in MIKE DeWINE any program, activity or facility, you should contact: The U. S. Fish and Wildlife Service Diversity & Civil Rights Programs-External Programs, DIRECTOR, OHIO DEPARTMENT 4040 N. -

08/19/2013 8:06 Am
ACTION: Original DATE: 08/19/2013 8:06 AM TO BE RESCINDED 1501:31-15-04 State-owned or administered lands designated as public hunting areas. (A) The following areas administered by divisions of the Ohio department of natural resources are hereby designated as public hunting areas upon which hunting and trapping are permitted as prescribed by the Revised Code or the Administrative Code. (1) The following state forests administered by the division of forestry: Blue Rock Brush creek Beaver creek Dean Fernwood Gifford Harrison Hocking Maumee Perry Pike Richland Furnace Scioto Trail Shade river Shawnee Sunfish creek Tar Hollow West Shade [ stylesheet: rule.xsl 2.14, authoring tool: i4i 2.0 ras3 Jan 10, 2013 11:43, (dv: 0, p: 109458, pa: 189439, ra: 348825, d: 438920)] print date: 08/19/2013 09:05 PM 1501:31-15-04 TO BE RESCINDED 2 Yellow creek Zaleski *Mohican Memorial, except for the following described two-hundred-seventy-acre tract: Beginning at the intersection of the center line of route 97 and the west edge of the air strip, thence along the west edge of the air strip continuing in a straight line across Pine Run to a point on the ridge where a westerly line will intersect the west line of the Memorial forest west of Pine Run and about one-half mile south of route 97, thence north with the forest boundary to the middle of route 97, thence with said center line to the point of beginning. (2) The following lands owned, administered by or under agreement with the division of wildlife are hereby designated as public hunting areas or wildlife areas: Acadia cliffs Aldrich pond Ales run American Electric Power recreational area Aquilla lake Auburn marsh Avondale Bayshore fishing access Beach City Beaver creek (Greene county) Beaver (Marion county) **B & N Coal, Inc. -
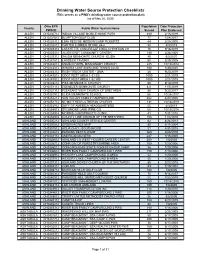
Drinking Water Source Protection Checklists (This Serves As a PWS's Drinking Water Source Protection Plan) (As of May 26, 2020)
Drinking Water Source Protection Checklists (This serves as a PWS's drinking water source protection plan) (as of May 26, 2020) Ohio EPA Population Date Protection County Public Water System Name PWS ID Served Plan Endorsed ALLEN OH0200712 INDIAN VILLAGE MOBILE HOME PARK 480 2/15/2019 ALLEN OH0231512 BLUFFTON GOLF CLUB 77 12/4/2017 ALLEN OH0232312 LIMA RESCUE MISSION/CAMP ROBERTS 44 2/28/2005 ALLEN OH0232812 CARTER LUMBER STORE 65-8 50 9/9/2019 ALLEN OH0233312 LAFAYETTE CONGREGATIONAL CHRISTIAN CH 70 9/16/2019 ALLEN OH0235412 ALLEN EAST COMMUNITY CENTER 190 2/28/2005 ALLEN OH0239312 SALEM MENNONITE CHURCH - ELIDA 80 6/5/2007 ALLEN OH0241812 LANDECK TAVERN 65 3/29/2005 ALLEN OH0242012 UNION CHAPEL MISSIONARY CHURCH 225 10/10/2014 ALLEN OH0243012 WINONA LAKE SWIM AND TENNIS CLUB 180 1/30/2020 ALLEN OH0244312 RUSH TRUCK CENTER - LIMA 40 9/23/2019 ALLEN OH0247812 ODOT REST AREA 1-31 EB 1000 2/21/2019 ALLEN OH0247912 ODOT REST AREA 1-32 WB 1000 2/21/2019 ALLEN OH0248212 PIKE MENNONITE CHURCH 60 9/10/2019 ALLEN OH0251112 EBENEZER MENNONITE CHURCH 421 11/5/2019 ALLEN OH0251312 PLEASANT VIEW CHURCH OF BRETHREN 86 9/22/2017 ALLEN OH0251912 ELIDA MENNONITE SCHOOL 66 10/26/2017 ALLEN OH0252212 SUN VALLEY FAMILY CAMPGROUND 482 4/27/2005 ALLEN OH0254712 ST MATTHEW LUTHERAN CHURCH 134 10/16/2019 ALLEN OH0254912 SAFY OF AMERICA HEADQUARTERS 55 4/3/2019 ALLEN OH0254913 SYCAMORE LAKE WINE CO 60 10/15/2014 ALLEN OH0254914 MORRIS CHIROPRACTIC CLINIC 41 1/31/2018 ALLEN OH0254916 COUNTY LINE CHURCH OF THE BRETHREN 158 11/8/2019 ASHLAND OH0300212 ASHLAND COUNTY -

Scouting in Ohio
Scouting Ohio! Sipp-O Lodge’s Where to Go Camping Guide Written and Published by Sipp-O Lodge #377 Buckeye Council, Inc. B.S.A. 2009 Introduction This book is provided as a reference source. The information herein should not be taken as the Gospel truth. Call ahead and obtain up-to-date information from the place you want to visit. Things change, nothing is guaranteed. All information and prices in this book were current as of the time of publication. If you find anything wrong with this book or want something added, tell us! Sipp-O Lodge Contact Information Mail: Sipp-O Lodge #377 c/o Buckeye Council, Inc. B.S.A. 2301 13th Street, NW Canton, Ohio 44708 Phone: 330.580.4272 800.589.9812 Fax: 330.580.4283 E-Mail: [email protected] [email protected] Homepage: http://www.buckeyecouncil.org/Order%20of%20the%20Arrow.htm Table of Contents Scout Camps Buckeye Council BSA Camps ............................................................ 1 Seven Ranges Scout Reservation ................................................ 1 Camp McKinley .......................................................................... 5 Camp Rodman ........................................................................... 9 Other Councils in Ohio .................................................................... 11 High Adventure Camps .................................................................... 14 Other Area Camps Buckeye .......................................................................................... 15 Pee-Wee ......................................................................................... -
FFY13 Ohio Nonpoint Source Management Program Annual Report
2013 Nonpoint Source Program FY13 Annual Report September 30, Big Walnut Creek Galena, Ohio Photo by Russ Gibson John Kasich, Governor Mary Taylor, Lt. Governor Scott Nally, Director TABLE OF CONTENTS Page Introduction ..................................................................................................................................................... 1 Program & Organizational Management ........................................................................................................ 1 Section 319(h) Grant Management ................................................................................................................. 4 FY09 Section 319(h) Grant Lake St. Marys Project ............................................................................... 9 FY11 Section 319(h) Buckeye Lake Nutrient Reduction Project ......................................................... 12 Statewide SWIF Grant Management ............................................................................................................. 16 FY10 Statewide SWIF Grant Management.......................................................................................... 17 FY12 Statewide SWIF Grant Management.......................................................................................... 18 Cuyahoga County GLRI-SWIF Grants ............................................................................................................. 21 FY12 Cuyahoga County GLRIL-SWIF Grant Management .................................................................. -

1501:31-15-04 AMENDMENT Rule Number TYPE of Rule Filing
ACTION: Original DATE: 02/03/2004 1:13 PM Rule Summary and Fiscal Analysis (Part A) Department Of Natural Resources Agency Name Division Of Wildlife Mindy Bankey Division Contact 1930 Belcher Drive Bldg. D-3 Columbus OH 614-265-6836 43224-1387 Agency Mailing Address (Plus Zip) Phone Fax 1501:31-15-04 AMENDMENT Rule Number TYPE of rule filing Rule Title/Tag Line State-owned or administered lands designated as public hunting areas. RULE SUMMARY 1. Is the rule being filed consistent with the requirements of the RC 119.032 review? No 2. Are you proposing this rule as a result of recent legislation? No 3. Statute prescribing the procedure in 4. Statute(s) authorizing agency to accordance with the agency is required adopt the rule: 1531.08 to adopt the rule: 119.03 5. Statute(s) the rule, as filed, amplifies or implements: 1531.08 6. State the reason(s) for proposing (i.e., why are you filing,) this rule: This rule regulates the state-owned or administered lands designated as public hunting areas. 7. If the rule is an AMENDMENT, then summarize the changes and the content of the proposed rule; If the rule type is RESCISSION, NEW or NO CHANGE, then summarize the content of the rule: Paragraph (A) has been amended and the and numbers "1234" have been deleted [ stylesheet: rsfa.xsl 2.05, authoring tool: EZ1, p: 13767, pa: 17070, ra: 58883, d: 62753)] print date: 02/03/2004 09:14 PM Page 2 Rule Number: 1501:31-15-04 and the words and numbers "1, 2, 3, 4,", "Bayshore fishing access" and "Muskingum watershed conservancy district" have been added. -

OGS FINDING AIDS 611 State Route 97 W Users of This Collection Should Credit the Ohio Genealogical Society in Any Reference Citing
OHIO GENEALOGICAL SOCIETY OGS FINDING AIDS 611 State Route 97 W Users of this collection should credit the Ohio Genealogical Society in any reference citing. The OGS Archives is open to Bellville OH 44813-8813 the public Tuesday through Saturday from 9 AM to 5 PM. In- 419-886-1903 quiries may also be made using the OGS Copy Service (Library section of web site) where a Manuscript Key and OGS Finding www.ogs.org Aids may also be found. MSS #145 Title: Joseph H. Shaw Ohio History Collection Bulk Dates: 1956-1971 Inclusive Dates: 1956-1971 Description: 2 phase boxes consisting of 11 files Organization: BOX 1 File 1: Water Transportation in Ohio Upper Ohio Valley Association. Facts Versus Fantasy: The High Cost of Cheap Transportation. 1961. [Pamphlet about the proposed Ohio River-Lake Erie Canal.] Stark County Historical Society. St. Helena II of Canal Fulton. [Pamphlet with map and schedule for passenger boat trips on the St. Helena II on the Ohio-Erie Canal] Northwest Ohio Great Lakes Research Center. Bowling Green State University. [Brochure about the holdings of the Great Lakes Research Center.] The Great Lakes Historical Society, 1965. Vermilion, Ohio. [Pamphlet] The Great Lakes Historical Society, >1965. Vermilion, Ohio. [Pamphlet, updated] Steamboats on the Western Waters. Campus Martius Museum, Marietta, Ohio. [Brochure, including pictures of the Fram- ing of the W.P. Snyder, Jr. and the W.P. Snyder, Jr. on the river at Pittsburgh] Canal Society of Ohio, Inc. [Membership pamphlet] Upper Ohio Valley Association. Facts about the Proposed Lake Erie-Ohio River Canal. 1957. -

Appendix E Conservation Lands Crossed by Nisource
APPENDIX E CONSERVATION LANDS CROSSED BY NISOURCE FACILITIES Appendix E – Conservation Lands Crossed by NiSource Facilities State Property Name Owner Type Delaware Bechtel Park Local Delaware Knollwood Park Local Delaware Naamans Park East Local Delaware Naamans Park North Local Indiana Eagle Lake Wetlands Conservation Area State Indiana Kingsbury Fish and Wildlife Area State Indiana Mallard Roost Wetland Conservation Area State Indiana St. John Prairie State Indiana Deep River County Park Local Indiana Northside Park Local Indiana Oak Ridge Prairie County Park Local Indiana Gaylord Butterfly Area NGO Kentucky Carr Creek State Park Federal Kentucky Daniel Boone National Forest Federal Kentucky Dewey Lake Wildlife Management Area Federal Kentucky Green River Lake Wildlife Management Area Federal Kentucky Jenny Wiley State Resort Park Federal Kentucky Lexington-Blue Grass Army Depot Federal Kentucky Carr Fork Lake Wildlife Management Area State Kentucky Central Kentucky Wildlife Management Area State Kentucky Dennis-Gray Wildlife Management Area State Kentucky Floracliff State Nature Preserve State Louisiana Bayou Teche National Wildlife Refuge Federal Louisiana Cameron Prairie National Wildlife Refuge Federal Louisiana Grand Cote National Wildlife Refuge Federal Louisiana Lacassine National Wildlife Refuge Federal Louisiana Mandalay National Wildlife Refuge Federal Louisiana Sabine National Wildlife Refuge Federal Louisiana Tensas River National Wildlife Refuge Federal Louisiana Big Lake Wildlife Management Area State Louisiana Boeuf Wildlife -

Ohio State Park Maps
Portage County Amateur Radio Service, Inc. (PCARS) 75 Ohio State Park Names and Ohio State Park Exchange Identifiers Ohio State Park Park ID Ohio State Park Park ID Adams Lake ADA Lake Loramie LOR Alum Creek ALU Lake Milton LML A.W.Marion AWM Lake White LWT Barkcamp BAR Little Miami LMI Beaver Creek BEA Madison Lake MLK Blue Rock BLU Malabar Farm MAL Buck Creek BCK Marblehead Lighthouse MHD Buckeye Lake BKL Mary Jane Thurston MJT Burr Oak BUR Maumee Bay MBY Caesar Creek CAE Middle Bass Island MBI Catawba Island CAT Mohican MOH Cowan Lake COW Mosquito Lake MST Deer Creek DEE Mt. Gilead MTG Delaware DEL Muskingum River MUS Dillon DIL Nelson Kennedy Ledges NKL East Fork EFK North Bass Island NBI East Harbor EHB Oak Point OPT Findley FIN Paint Creek PTC Forked Run FOR Pike Lake PLK Geneva GEN Portage Lakes POR Grand Lake St. Marys GLM Punderson PUN Great Seal GSL Pymatuning PYM Guilford Lake GLK Quail Hollow QHL Harrison Lake HLK Rocky Fork RFK Headlands Beach HEA Salt Fork SFK Hocking Hills HOC Scioto Trail STR Hueston Woods HUE Shawnee SHA Independence Dam IDM South Bass Island SBI Indian Lake ILK Stonelick STO Jackson Lake JAC Strouds Run SRN Jefferson Lake JEF Sycamore SYC Jesse Owens JEO Tar Hollow TAR John Bryan JOB Tinker’s Creek TCK Kelleys Island KEL Van Buren VAN Kiser Lake KLK West Branch WBR Lake Alma LAL Wingfoot Lake WLK Lake Hope LHO Wolf Run WRN Lake Logan LOG OSPOTA Park IDs - Jan 2019 Ohio State Parks On The Air LOCATION MAP LEGEND Adams Lake State Park SR 32 SR 23 Park Office Park location: SR 41 Adams Lake Picnic Area 14633 State Route 41 State Park Picnic Shelter West Union, Ohio 45693 WEST UNION Restroom SR 247 PORTSMOUTH SR 125 Boat Launch GPS Coordinates: o Hiking Trail 38 44’ 28.83” N US 52 Shawnee State Park 83o 31’ 12.48” W Park Boundary OHIO RIVER State Nature Preserve Waterfowl Hunting Area KENTUCKY Park Road 2 (Lake Drive) Administrative office: Shawnee State Park 4404 State Route 125 West Portsmouth, Ohio 45663-9003 (740) 858-6652 - Shawnee Park Office Spillway ADAMS LAKE Lick Run Rd. -

Nwh En Buckeye Lake Dam REVIEW of PAST REPORTS and EXISTING CONDITIONS and RECOMMENDATIONS for INTERIM RISK REDUCTION MEASURES
NWh Buckeye Lake Dam en Fairfield and Licking Counties, Ohio REVIEW OF PAST REPORTS AND EXISTING CONDITIONS AND RECOMMENDATIONS FOR INTERIM RISK REDUCTION MEASURES, OPERATION, AND MAINTENANCE Huntington District, Great Lakes and Ohio River Division March 2015 Status: Final i CONTENTS Executive Summary .............................................................................................................................................................. III 1 Introduction ..................................................................................................................................................................... 1 1.1 ODNR-Army Corps of Engineers Scope of Work ..................................................................................... 1 1.2 Background of Buckeye Lake Dam ................................................................................................................ 1 1.3 Project Description .............................................................................................................................................. 2 1.4 Past USACE High Pool Performance Observations ................................................................................. 3 2 Review of Previous Engineering Consultant Reports ..................................................................................... 4 2.1 Summary of Findings from Consultant Reports ...................................................................................... 4 2.2 Evaluations of Consultant Reports ............................................................................................................... -

Assigning Stream Use Designations for the Protection of Recreational
Ohio EPA Assigning Stream Use Designations for the Policy Protection of Recreational Uses DSW-0700.008 Ohio EPA, Division of Surface Water Statutory references: Revision 0, February 21, 1985 Rule references: Revision 1, September 30, 1999 Removed Removed, December 21, 2006 THIS POLICY DOES NOT HAVE THE FORCE OF LAW Pursuant to Section 3745.30 of the Revised Code, this policy was reviewed and removed. This policy does not meet the definition of policy contained in Section 3745.30 of the Ohio Revised Code. Ohio EPA is removing this document from the Division of Surface Water Policy Manual and is considering addressing this topic in a future rulemaking. For more information contact: Ohio EPA, Division of Surface Water Standards and Technical Support Section P.O. Box 1049 Columbus OH 43216-1049 (614) 644-2001 H:\RulePolicyGuid_Effective\Policy\2006ManualUpdate\07_08r.wpd Ohio EPA Assigning Stream Use Designations for Policy the Protection of Recreational Uses DSW-0700.008 Ohio EPA, Division of Surface Water Statutory reference: ORC 6111.041 Revision 0, February 21, 1985 Final Rule reference: OAC 3745-1-07(B)(4) Revision 1, September 30, 1999 THIS POLICY DOES NOT HAVE THE FORCE OF LAW Pursuant to Section 3745.30 of the Revised Code, this policy was reviewed on the last revision date. Purpose To identify the guidelines Ohio EPA will use to designate water quality standard recreational uses to surface waters of the State. Background The recreational season in Ohio is the time period from May 1 to October 15. During this period surface waters must meet numerical criteria which protect the recreational use. -

03/25/2010 9:15 Am
ACTION: Original DATE: 03/25/2010 9:15 AM 1501:41-15-08 Parks wherein all legal game may be hunted. It shall be lawful to hunt game birds, game quadrupeds, and fur bearing animals and trap fur bearing animals in the parks named in this rule when done in accordance to the official hunting maps for the parks listed. Official hunting maps indicate those areas of the park open and closed to hunting and trapping and any restrictions on hunting or trapping in those areas. The official copies of the maps are filed with the chief of the division. At least thirty days prior to the effective date copies of the maps are posted in accordance with rule 1501:41-3-01 of the Administrative Code and filed at the respective area headquarters office. Parks that have areas open to hunting and trapping: Adams Lake state park Alum Creek state park A.W. Marion state park Barkcamp state park Beaver Creek state park Blue Rock state park Buck Creek state park Buckeye lake state park Burr Oak state park Caesar Creek state park Cowan lake state park Crane Creek state park Deer Creek state park Delaware state park Dillon state park East Fork state park East Harbor state park Findley state park [ stylesheet: rule.xsl 2.14, authoring tool: i4i 2.0 Apr 9, 2003, (dv: 1, p: 62777, pa: 106299, ra: 230232, d: 278139)] print date: 03/25/2010 09:01 PM 1501:41-15-08 2 Forked Run state park Geneva state park Grand lake St. Marys state park Great Seal state park Guilford Lake state park Hocking Hills state park Hueston Woods state park Indian lake state park Jackson lake state