Characterisation of the Immune Responses of the Koala
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
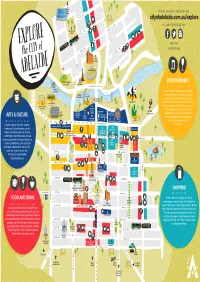
Cityofadelaide.Com.Au/Explore RYMILL NATIONAL NATIONAL
TO LE FEVRE TCE ADELAIDE AQUATIC FOR TIPS ON WHAT TO SEE AND DO VISIT CENTRE TYNTE ST cityofadelaide.com.au/explore FOLLOW CITYOFADELAIDE ON: WELLINGTON KINGSTON TCE SQUARE ARCHER ST O’CONNELL ST STANLEY ST #ADELAIDE #VISITADELAIDE WARD ST MELBOURNE ST ST PETER’S CATHEDRAL JEFFCOTT ST ADELAIDE OVAL NORTH ADELAIDE COLONEL ENTERTAINMENT GOLF COURSE LIGHT STATUE WAR MEMORIAL DRIVE TORRENS RIVER WEIR Wander through the yellow areas of the map TORRENS for a range of things to see and do for all ages, FOOTBRIDGE ROTUNDA including music, comedy, theatre, sport and POPEYE ELDER PARK ADELAIDE ZOO recreation. Check out a game at the Adelaide Oval, OLD ADELAIDE LAUNCH GAOL BONYTHON PARK take a cruise along the River Torrens, play a round PLAYSPACE ADELAIDE CASINO FESTIVAL at the North Adelaide Golf Course, visit the GOVERNMENT NATIONAL CENTRE ADELAIDE HOUSE MIGRATION WINE CENTRE Adelaide Zoo or catch a live band. There is SAHMRI CONVENTION NATIONAL MUSEUM RAILWAY ARTS & CULTURE CENTRE WAR MEMORIAL always something entertaining happening STATION BOTANIC PARLIAMENT AVE KINTORE ART GARDENS HOUSE LIBRARY MUSEUM GALLERY in the City, day and night and all NORTH TCE year round! A diverse range of museums, galleries, SAMSTAG MUSEUM LION ARTS CENTRE theatres and cultural landmarks can be MALLS BALLS AYERS HOUSE MALLS PIGS JAM FACTORY ST FROME found in the dark blue areas of the map. MERCURY CINEMA BANK ST GAWLER PL GAWLER ST PULTENEY HINDLEY ST MEDIA RESOURCE CENTRE A stroll through any of these areas will also RUNDLE MALL uncover quirky street art, famous statues and RUNDLE ST sculptures. -

Event Information
BLANCO FOOD & EVENTS: RESTAURANT & CATERING AWARDS FOR EXCELLENCE National Event Caterer of the Year 2008 South Australian Event Caterer of the Year 2003, 2004, 2005, 2007, 2008, 2009 ENQUIRIES T. +61 8 8230 1313 PO Box 2669 South Australian Caterer of the Year 2003 [email protected] F. +61 8 8132 0813 Kent Town South Australian Hall of Fame 2006, 2010 www.blancofood.com.au South Australia 5071 South Australian Sanctuary Adelaide Zoo – Venue Caterer of the Year 2010, 2011 EVENT INFORMATION PLANE TREE DRIVE, ADELAIDE, SOUTH AUSTRALIA A BLANCO FOOD & EVENTS VENUE www.blancofood.com.au | [email protected] | +61 8 8230 1313 WELCOME Thank you for considering the Sanctuary Adelaide Zoo and the award winning Blanco Food & Events Team for your upcoming event. Opened in 2010, the Sanctuary Adelaide Zoo is a state of the art function facility located on the first floor of the Adelaide Zoo’s new $30 million re-development. This development has coincided with the arrival of Wang Wang and Funi – the only two giant pandas in the southern hemisphere. Sanctuary Adelaide Zoo provides the latest technology within flexible meeting, exhibition or banquet space over looking the magnificent parklands of Adelaide’s CBD fringe. Managed by Blanco Food & Events, our professional service and depth of experience is reflected in our multiple awards including “The Best Achievement in Catering” at the 2011 Australian Event Awards. Blanco’s partnership with Adelaide Zoo provides you with amazing animal encounters at your event which are unequalled. Giant pandas, tiger feeding, animal handling, can provide a memorable experience at your event. -

Download the World Routes 2019 Essential Guide to Adelaide
Your Essential Guide to Adelaide World Routes 2019 | 21 - 2 4 S eptember I A world of experiences at your fingertips in Adelaide. Adelaide is bursting with culture, flavours, events and entertainment. This vibrant and friendly city invites you to reward your curiosity and discover what makes Adelaide the perfect home for World Routes 2019. Adelaide Oval, Adelaide Your Essential Guide Welcome to Adelaide Welcome, from the hosts of World Routes 2019. Surrounded by lush parklands and speckled with an eclectic combination of historic buildings, trendy bars and state-of- the-art modern facilities, Adelaide is beckoning to be explored. We invite you to indulge in some of Australia’s most awarded restaurants in the heart of the city, immerse yourself in a thriving local arts scene, and unveil the oldest culture on earth through the world’s largest Aboriginal artefact collection. Adelaide, with its bustling Riverbank Precinct, and world-class venues such as the Adelaide Oval, Adelaide Showground and Adelaide Convention Centre, is an ideal setting for major events and conferences. The city can accommodate event-goers from around the world but remains compact enough to enable our visitors to roam freely, explore local attractions, and stay confident that their home base is never too far. Step outside of the city, soak up some sun and uncover a diverse array of experiences in our regions. Taste your way through world-famous wine regions only minutes from the city. Adelaide is a gateway to some of Australia’s best wine country and is recognised as a member of the prestigious Great Wine Capitals Global Network. -

Immune-Inflammatory Concept of the Pathogenesis of Chronic Heart Failure in Dogs with Dilated Cardiomyopathy
Veterinary World, EISSN: 2231-0916 RESEARCH ARTICLE Available at www.veterinaryworld.org/Vol.12/September-2019/21.pdf Open Access Immune-inflammatory concept of the pathogenesis of chronic heart failure in dogs with dilated cardiomyopathy Yu Vatnikov1, A. Rudenko2, P. Rudenko3, Ev Kulikov1, A. Karamyan1, V. Lutsay2, I. Medvedev4, V. Byakhova1, E. Krotova1 and M. Molvhanova1 1. Department of Veterinary Medicine, Peoples’ Friendship University of Russia (RUDN University), Moscow 117198, Russia; 2. Department of Veterinary Medicine, Moscow State University of Food Production, Moscow 125080, Russia; 3. Laboratory of Biological Experiments, Branch of the Shemyakin-Ovchinnikov Institute of Bioorganic Chemistry of the Russian Academy of Sciences, Pushchino 117997, Russia; 4. Department of Adaptive Physical Culture and Recreation, Russian State Social University, Moscow 129226, Russia. Corresponding author: V. Byakhova, e-mail: [email protected] Co-authors: YV: [email protected], AR: [email protected], PR: pavelrudenko76@yandex. ru, EvK: [email protected], AK: [email protected], VL: [email protected], IM: [email protected], EK: [email protected], MM: [email protected] Received: 17-02-2019, Accepted: 07-08-2019, Published online: 28-09-2019 doi: 10.14202/vetworld.2019.1491-1498 How to cite this article: Vatnikov Y, Rudenko A, Rudenko P, Kulikov E, Karamyan A, Lutsay V, Medvedev I, Byakhova V, Krotova E, Molvhanova M (2019) Immune-inflammatory concept of the pathogenesis of chronic heart failure in dogs with dilated cardiomyopathy, Veterinary World, 12(9): 1491-1498. Abstract Background: Dilated cardiomyopathy is common in dogs. This form of cardiomyopathy is the main cause of death due to heart disease in dogs. -

High School Formal Packages
BLANCO FOOD & EVENTS: RESTAURANT & CATERING AWARDS FOR EXCELLENCE National Event Caterer of the Year 2008 South Australian Event Caterer of the Year 2003, 2004, 2005, 2007, 2008, 2009 ENQUIRIES T. +61 8 8230 1313 PO Box 2669 South Australian Caterer of the Year 2003 [email protected] F. +61 8 8132 0813 Kent Town South Australian Hall of Fame 2006, 2010 www.blancofood.com.au South Australia 5071 South Australian Sanctuary Adelaide Zoo – Venue Caterer of the Year 2010, 2011 South Australian Tourism Restaurant of the Year 2012 HIGH SCHOOL FORMAL PACKAGES PLANE TREE DRIVE, ADELAIDE, SOUTH AUSTRALIA A BLANCO FOOD & EVENTS VENUE www.blancofood.com.au | [email protected] | +61 8 8230 1313 WELCOME Opened in 2010, the Sanctuary Adelaide Zoo is a state of the art function facility located on the first floor of the Adelaide Zoo’s new $30 million re-development. Book your This development has coincided with the arrival of Wang Wang and Funi, the only two giant pandas in the southern School formal by hemisphere. March 2013 for Sanctuary Adelaide Zoo offers flexible banquet space for dining and cocktail style within the magnificent parklands of 2013 and receive Adelaide’s CBD fringe. Managed by Blanco Food Events, our depth of experience is gift prizes of: reflected in our constant return clientele and multiple awards. We are Restaurant and Catering, Venue Caterer of the Year 2010 and 2011 and Events Australia Best Achievement in Catering 2011. We are dedicated to making your school ● 3 Breakfast formal unique and special with the packages we offer. -

Zoo Note Koalas Are Arboreal, Which Means They Are Tree in Queensland and New South Wales Are Smaller Dwellers
KOALA s The koala (Phascolarctos cinereus), often mistaken non-eucalypt species when eucalypts are sparse. for a bear, is unique to Australia. It is a marsupial Koalas obtain sufficient water from their food and ADELAIDE ZOO whose closest relation is the wombat. Fossil therefore rarely leave the trees to drink. They EDUCATION SERVICE ancestors of the Koala date back 15 million years. will drink however, when it is hot and water is The name ‘Koala’ is derived from an Aboriginal term available. translated to ‘the animal that does not drink’. The natural life of a Koala spans between 15 and 18 Koalas found in South Eastern Australia are fairly years. robust (adult males average 11.8 kg, females 7.9kg) with dark grey, shaggy fur. Koalas found zoo note Koalas are arboreal, which means they are tree in Queensland and New South Wales are smaller dwellers. Koalas are folivores (foliage eaters), (adult males average 6.5kg, females 5.1 kg) with feeding on the leaves of eucalypt trees. They move reddish or tawny fur. The Koalas at the Adelaide around and feed at night with peak activity being Zoo are native to Victoria. just after sunset. An adult consumes up to 1 kilogram of leaves per day and it must spend up to Most marsupials have a pouch, (some only have 19 hours per day sleeping in the fork of a tree to a flap of skin), designed for rearing their young, allow digestion to occur. Eucalypt leaves provide a which opens upwards. However, a Koala’s pouch high fibre, low protein diet and the koala’s digestive faces downward, like a wombat’s. -

Top 10 Things to Do in Adelaide
FACT SHEET TOP 10 THINGS TO DO IN ADELAIDE Explore Adelaide’s attractions and experiences in this list of top 10 things to do. Nestled between the hills and the ocean, PORT ADELAIDE Adelaide is quickly becoming the lifestyle capital of Australia. Already celebrated for Discover Port Adelaide’s wealth of 19th- its wine and food culture, this elegant city century buildings, classic Australian pubs is undergoing a rapid transformation with and atmospheric old wharves. Pick up a self- a burgeoning small-bar scene, world-class guided walking map from the tourist office, festivals and amazing eco-adventures. kayak along Port River (home to a dolphin colony) or drop into the Maritime Museum on Lipson Street. Afterwards, visit the Port LANEWAY BARS Dock Brewery Hotel for a craft beer or two. Stroll through Adelaide’s laneways where warehouses and shops are being NORTH TERRACE transformed into bars serving everything from tapas to Serbian cuisine. Hit the Visit Adelaide’s most elegant boulevard, Peel-Leigh Street precinct, kicking off North Terrace, home to the city’s most with a wine at Clever Little Tailor before important cultural institutions. Apart from dropping in BarBushka for a cocktail. State Parliament, Adelaide Casino and the Don’t miss Proof, a buzzy little joint. Convention Centre, this tree-lined street hosts the Art Gallery of South Australia, the South Australian Museum and The ADELAIDE CENTRAL MARKET University of Adelaide. Round off your walk Marvel at South Australia’s rich bounty by visiting the Botanic Gardens, a legacy of produce – from farm-fresh fruit and of Adelaide’s past with historic buildings vegetables to hormone-free meats, artisan and three striking glasshouses on site. -

CBD VENUES & ACCOMMODATION Nestled Within a Park Adelaide’S One Square Mile City Ensures That Hotels and Venues Are All Closely Located
DIRECTORY VENUES, SERVICES & ACCOMMODATION South Australia boasts professional and experienced business event suppliers who are committed to making your event a success. 30 COVID-19 UPDATE Due to COVID-19, some information here might not be up-to-date. To find out the latest on business events in South Australia, please contact the Adelaide Convention Bureau directly. +61 8 8237 0100 adelaideconvention.com.au [email protected] 2 Adelaide CBD VENUES & ACCOMMODATION Nestled within a park Adelaide’s one square mile city ensures that hotels and venues are all closely located. 31 CBD MAP CBD VENUES, LOCATIONS & ACCOMMODATION ENTERTAINMENT PRECINCT CBD VENUES 2 1. Adelaide Convention Centre BUSINESS PRECINCT 2. Adelaide Entertainment Centre 4km from Adelaide CBD 7 3. Adelaide Festival Centre KING WILLIAM STREET 4. Adelaide Oval Functions & Events 4 5. Ayers House CULTURAL PRECINCT 6. National Wine Centre of Australia BIOMED CITY 3 7. Sanctuary Adelaide Zoo Functions & Events Centre CBD ACCOMMODATION 3 1. Adelaide Casino 6 2. Avani Adelaide Residences 3. Hilton Adelaide 4 1 6 1 7 6 2 4. Holiday Inn Express 5 5. Hotel Grand Chancellor 9 10 8 15 14 4 6. InterContinental Adelaide 5 RUNDLE STREET HINDLEY STREET 7. Mayfair Hotel 5 7 8. Mercure Grosvenor Hotel 12 9. Oaks Embassy 10. Oaks Horizons 11. Oaks Plaza Pier 10.6km from Adelaide CBD 12. Pullman Adelaide 2 16 13. Stamford Grand 11.1km from Adelaide CBD Adelaide Airport WAKEFIELD ROAD 14. Stamford Plaza Only 10 mins drive WAKEFIELD STREET GROTE STREET 15. The Playford from the CBD! WEST TERRACE 1 3 16. -

Say at Adelaide
adelaidezoo.com.au Say ‘Iat Adelaidedo’ Zoo Adelaide Zoo is part of Zoos SA; a not-for-profit zoo-based conservation charity. We exist to save species from extinction and connect people with nature and as part of our conservation efforts we run Adelaide and Monarto Zoos. We rely on the generous support from the community to care for endangered animals, undertake research and conservation programs and delight and educate zoo visitors. Central Lawn Garden Ceremonies Package includes • Entry into the Zoo for up to 120 people • Welcome signage to greet your guests • Choice of 3 Adelaide Zoo garden ceremony locations – the Central Lawns, the Jewels of Asia Lawns or the Immersion Lawns Immersion Lawn • An Adelaide Zoo host to coordinate your ceremony • 16 white Americana chairs • Clothed signing table and two chairs • Separate entrance for the wedding party • Car parking for 1 car • Complimentary wet weather back up venue (subject to availability) • Access to the zoo for a wedding rehearsal (by appointment) From 1 Jan 2017 price - $1090 From 1 July 2018 price - $1149 * After 5pm Wedding Packages also available Jewels of Asia Lawn Rotunda Ceremony Rotunda package includes • Entry into the Zoo for up to 120 people • Welcome signage to greet your guests • An Adelaide Zoo host to coordinate your ceremony • 16 white Americana chairs • Clothed signing table and two chairs • Separate entrance for the wedding party • Car parking for 1 car • Access to white umbrellas if required for wedding party • Access to the zoo for a wedding rehearsal (by appointment) From 1 Jan 2017 price - $1350 From 1 July 2018 price - $1420 * After 5pm Wedding Packages also available Bamboo Forest Ceremony Package includes Say your vows with Wang Wang and Funi as your witnesses! • The Panda Keeper will be on site to give a presentation or answer questions. -

Clinical and Molecular Aspects of Severe Malaria
Anais da Academia Brasileira de Ciências (2005) 77(3): 455-475 (Annals of the Brazilian Academy of Sciences) ISSN 0001-3765 www.scielo.br/aabc Clinical and molecular aspects of severe malaria KARIN KIRCHGATTER1 and HERNANDO A. DEL PORTILLO2 1Núcleo de Estudos em Malária, Superintendência de Controle de Endemias (SUCEN)/ Instituto de Medicina Tropical de São Paulo (IMTSP), Universidade de São Paulo (USP) 05403-000 São Paulo, SP, Brasil 2Departamento de Parasitologia, Instituto de Ciências Biomédicas, Universidade de São Paulo (USP) 05508-900 São Paulo, SP, Brasil Manuscript received on March 3, 2005; accepted for publication on March 28, 2005; presented by George A. DosReis ABSTRACT The erythrocytic cycle of Plasmodium falciparum presents a particularity in relation to other Plasmodium species that infect man. Mature trophozoites and schizonts are sequestered from the peripheral circulation due to adhesion of infected erythrocytes to host endothelial cells. Modifications in the surface of infected erythrocytes, termed knobs, seem to facilitate adhesion to endothelium and other erythrocytes. Adhesion provides better maturation in the microaerophilic venous atmosphere and allows the parasite to escape clear- ance by the spleen which recognizes the erythrocytes loss of deformability. Adhesion to the endothelium, or cytoadherence, has an important role in the pathogenicity of the disease, causing occlusion of small vessels and contributing to failure of many organs. Cytoadherence can also describe adhesion of infected erythrocytes to uninfected erythrocytes, a phenomenon widely known as rosetting. Clinical aspects of severe malaria, as well as the host receptors and parasite ligands involved in cytoadherence and rosetting, are reviewed here. The erythrocyte membrane protein 1 of P. -

1.4 Life Cycle of Plasmodium Falciparum
Programmierter Zelltod in Plasmodium infizierten HbA/A und HbA/S Erythrozyten Programmed Cell Death in Plasmodium Infected Normal and Sickle Trait Red Blood Cells DISSERTATION der Fakultät für Chemie und Pharmazie der Eberhard-Karls-Universität Tübingen zur Erlangung des Grades eines Doktors der Naturwissenschaften 2007 vorgelegt von Verena Beatrice Brand Tag der mündlichen Prüfung: 30. August 2007 Dekan: Prof. Dr. L. Wesemann 1. Berichterstatter Prof. Dr. F. Lang 2. Berichterstatter Prof. Dr. M. Duszenko 2 CONTENTS______________ ____________________________________________________ Contents ACKNOWLEDGMENTS 8 LIST OF FIGURES AND TABLES 10 LIST OF ABBREVIATIONS 13 1 INTRODUCTION 16 1.1 Impact and distribution of malaria 16 1.2 Discovery of Plasmodium 17 1.3 Evolution of Plasmodium spp. 17 1.4 Life cycle of Plasmodium falciparum 18 1.4.1 The arthropod vector 19 1.4.1.1 Sporogony 19 1.4.2 Merogony in the liver 20 1.4.3 Erythrocytic cycle: Disease 21 1.4.3.1 Invasion of erythrocytes by merozoites 21 1.4.3.2 Asexual replication: trophozoites and schizontes 22 1.4.4 Gametocytogenesis 25 1.5 Development of resistance towards antimalarial drugs 25 1.6 Erythrocyte ion composition and regulation 26 1.6.1 Active ion transport 27 1.6.2 Na +/K + pump-leak balance in non-infected erythrocytes 27 1.6.3 Ca 2+ homeostasis in non-infected erythrocytes 28 1.6.4 Nonselective cation channels in non-infected erythrocytes 28 1.6.5 Ca 2+ activated Gardos K + channels 29 1.7 Functional significance of the nonselective cation channels, Ca 2+ signaling, and Gardos channel activity for the volume and programmed death of erythrocytes 31 1.7.1 Erythrocyte death signaling pathways 31 1.7.1.1 The role of nonselective cation channels in eryptosis upon PGE 2 formation 33 1.7.1.1.1 Activation of lipid transporters involved in phosphatidylserine movement 34 1.7.2 Recognition of phosphatidylserine-exposing erythrocytes by macrophages 36 3 CONTENTS______________ ____________________________________________________ 1.8 P. -

The Rheology of Severe Falciparum Malaria W
UvA-DARE (Digital Academic Repository) On the pathophysiology of severe falciparum malaria with special reference to red cell deformability Dondorp, A.M. Publication date 1999 Link to publication Citation for published version (APA): Dondorp, A. M. (1999). On the pathophysiology of severe falciparum malaria with special reference to red cell deformability. General rights It is not permitted to download or to forward/distribute the text or part of it without the consent of the author(s) and/or copyright holder(s), other than for strictly personal, individual use, unless the work is under an open content license (like Creative Commons). Disclaimer/Complaints regulations If you believe that digital publication of certain material infringes any of your rights or (privacy) interests, please let the Library know, stating your reasons. In case of a legitimate complaint, the Library will make the material inaccessible and/or remove it from the website. Please Ask the Library: https://uba.uva.nl/en/contact, or a letter to: Library of the University of Amsterdam, Secretariat, Singel 425, 1012 WP Amsterdam, The Netherlands. You will be contacted as soon as possible. UvA-DARE is a service provided by the library of the University of Amsterdam (https://dare.uva.nl) Download date:01 Oct 2021 n zr &> CD -5 The rheology of severe falciparum malaria W A.M. Dondorp, P.A. Kager, J. Vreeken, N.J. White Department of Internal Medicine and Division of Infectious Diseases, Tropical Medicine and AIDS, Academic Medical Centre, Amsterdam, the Netherlands (A.M. Dondorp, MD, Prof. P.A. Kager, MD, PhD, Prof. J.