The Effects of Intraspecific, Interspecific, and Intergeneric Grafting on the Growth and Development of Fraxinus Excelsior Scions
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Technical Note 30: Ability of Pacific Northwest Shrubs to Root From
TECHNICAL NOTES _____________________________________________________________________________________________ U.S. DEPT. OF AGRICULTURE NATURAL RESOURCES CONSERVATION SERVICE PORTLAND, OREGON DATE: September 2002 PLANT MATERIALS TECHNICAL NOTE NO. 30 Ability of Pacific Northwest Native Shrubs to Root from Hardwood Cuttings (with Summary of Propagation Methods for 22 Species) Dale C. Darris Conservation Agronomist Corvallis Plant Materials Center SUMMARY There is a need for additional information on how well native shrubs root from hardwood cuttings. First, nurseries are seeking to refine vegetative propagation techniques. Second, practitioners in the fields of riparian/wetland restoration and streambank stabilization are looking for species that are easy to root from unrooted dormant material that will provide cost competitive alternatives to native willows (Salix spp.) and redosier dogwood (Cornus sericea) for direct sticking of cuttings and soil bioengineering practices. The purpose of this work was to screen 15 shrubs indigenous to the Pacific Northwest USA for their ability to root from hardwood cuttings with and without a rooting compound in a greenhouse mist bench, well drained field, nursery bed, and saturated substrate (artificial pond). If a species roots easily, it has greater potential for planting as dormant cuttings, live stakes, or whips directly on a revegetation site, and may warrant further evaluation for fascines, brush mattresses, or other soil bioengineering practices. Based on the rooting ability of hardwood cuttings made from 1 (or 2) year old wood, those species with good potential include black twinberry (Lonicera involucrata), salmonberry (Rubus spectabilis), common snowberry (Symphoricarpos albus), and Pacific ninebark (Physocarpus capitatus). Coyote brush (Baccharis pilularis) and red elderberry (Sambucus racemosa) have fair potential. Indian plum (Oemlaria cerasiformis), mock orange (Philadelphus lewisii), and red flowering currant (Ribes sanguineum) may apply under limited circumstances, select uses, or ideal conditions. -

Salt-Spray Tolerant Groundcovers, Shrubs, and Trees for Eastern Long Island
Salt-spray Tolerant Groundcovers, Shrubs, and Trees for Eastern Long Island Aerial salts carried by on-shore breezes, fog, and wind can injure plants sensitive to salt deposition. The plants listed below have displayed either high or moderate tolerance to salt spray. Most species listed have displayed high tolerance ( little to no damage). Those species noted as having moderate tolerance may show signs of salt injury (tip dieback, foliar damage, reduced growth). Moderately-tolerant species should be planted in areas away from direct salt-spray exposure. Salt-spray tolerance applies to aerial deicing salts as well. The plants listed were chosen because they are relatively disease and pest resistance (unless noted), and well-suited for eastern Long Island. Perennial Groundcovers Foliage Common Name Scientific Name Habit & Comments* Deciduous Beach Pea Lathyrus maritimus native, flowering, silver-green foliage Evergreen Bearberry Arctostaphylos uva-ursi native, low-growing, soft stems Evergreen Shore Juniper Juniperus conferta spreading, dense Semi-evergreen Willowleaf Cotoneaster Cotoneaster salicifolius weeping, cascading Semi-evergreen Lily Turf Liriope muscari grass-like appearance Woody Shrubs (fifteen feet or less in height) Deciduous False Indigo-bush Amorpha fruticosa flowering shrub, prefers wet sites Deciduous Red Chokeberry Aronia arbutifolia moderate salt tolerance, native, early flowering Deciduous Black Chokecherry Aronia melanocarpa moderate salt tolerance, native, early flowering Deciduous Eastern Baccharis Baccharis halimifolia native, tolerates wet sites Deciduous Sweet Pepperbush Clethra alnifolia native, flowers late Deciduous Sweetfern Comptonia peregrina native, prefers dry sites Deciduous Creeping Cotoneaster Cotoneaster adpressus mounded, spreading Deciduous Cotoneaster Cotoneaster divaricatus upright, arching habit Deciduous Forsythia Forsythia x intermedia early spring flowering Deciduous Common Witchhazel Hamamelis virginiana native, very early flowering Deciduous Rose of Sharon Hibuscus syriacus moderately salt tolerant, flowering, mod. -
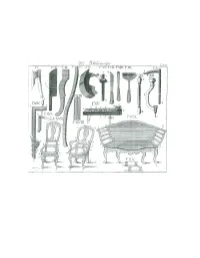
Tools of the Cabinetmaker, but Also Like the Cartwright, the Hatchet (Handbeil) and the Drawknife (Schneidemesser)
CHAPTER FIVE The Chairmaker The chairmaker bears the name in common with English chairmakers presumably because his trade is originally transplanted from England to Germany, or because several types of chairs that are made in his workshop have been common first in England. In the making of chairs, the settee (Canape), and sofa, he wields not only the plane and other tools of the cabinetmaker, but also like the cartwright, the hatchet (Handbeil) and the drawknife (Schneidemesser). I. In most regions, and especially in the German coastal cities, chairmakers make their chairs out of red beech wood, in Magdeburg out of linden wood, and in Berlin out of serviceberry wood (Elsenholz). Red beech is lacking in our area, and the cabinetmaker, who before the arrival to Berlin of chairmakers that made wooden chair frames, chose therefore serviceberry wood in place of red beech. Likewise the chairmakers, when they arrived in Berlin, found that circumstances also compelled them to build their chairs out of serviceberry wood. If the customer explicitly requires it, and will pay especially for it, they sometimes build chairs out of walnut, plum wood, pearwood, and mahogany wood, and for very distinguished and wealthy persons out of cedarwood. The chairmaker obtains the serviceberry wood partly in boards that are one to five inches thick and partly in logs. The farmer in the [town of] Mark Brandenburg brings this wood, partly in logs and also in boards, to Berlin to sell, but the strongest and best comes from Poland. If the wood has not sufficiently dried when purchased by the chairmaker it must stay some time longer and properly dry. -

Verticillium Wilt of Fraxinus Excelsior
Verticillium wilt of Fraxinus excelsior - ' . ; Jt ""* f- "" UB-^/^'IJ::J CENTRALE LANDBOUWCATALOGUS 0000 0611 8323 locjs Promotoren: Dr.Ir. R.A.A. Oldeman Hoogleraar ind e Bosteelt en Bosoecologie Dr.Ir. J. Dekker Emeritus Hoogleraar ind e Fytopathologie /OMOS-Zöl,^ Jelle A. Hiemstra Verticillium wilt of Fraxinus excelsior Proefschrift ter verkrijging van de graad van doctor in de landbouw- en milieuwetenschappen, op gezag van de rector magnificus, dr. C.M. Karssen, inhe t openbaar te verdedigen op dinsdag 18apri l 1995 des namiddags omvie r uur ind e aula van de Landbouwuniversiteit te Wageningen J\ ABSTRACT Hiemstra, J.A. (1995). Verticillium wilt of Fraxinus excelsior. PhD Thesis, Wageningen Agricultural University, The Netherlands, xvi + 213 pp, 40 figs., 28 tables, 4 plates with colour pictures, 327 refs., English and Dutch summaries. ISBN 90-5485-360-3 Research on ash wilt disease, a common disease of Fraxinus excelsior L. in young forest and landscape plantings in several parts of the Netherlands, is described. By means of a survey for pathogenic fungi in affected trees, inoculation and reisolation experimentsi ti sdemonstrate d thatth ediseas ei scause db y Verticilliumdahliae Kleb . Hostspecificit y andvirulenc e of aV. dahliae isolatefro m ashar ecompare d tothos e of isolatesfro m elm,mapl ean dpotato .Diseas eincidenc ean dprogress , andrecover y of infected trees are investigated through monitoring experiments in two permanent plots in seriously affected forest stands. Monitoring results are related to the results of an aerial survey for ash wilt disease in the province of Flevoland to assess the impact of the disease on ash forests. -

Regional Woody Plant Test Project 2005
Regional Woody Plant Test Project 2005 CDCS Crop Diversification Centre South Brooks, Alberta Pamphlet #2006-3 Regional Woody Plant Test Project 2005 Christine L. Murray, Ph.D., Nursery Crop Specialist Nigel G. Seymour, Dipl. Hort. Technologist Alberta Agriculture, Food and Rural Development Crop Diversification Centre South SS 4, Brooks, Alberta, Canada T1R 1E6 email: [email protected] Phone (403) 362-1313 Fax (403) 362-1306 [email protected] Phone (403) 362-1350 Fax (403) 362-1306 website: http://www1.agric.gov.ab.ca/$department/deptdocs.nsf/all/opp4045?opendocument Table of Contents Index - Botanical Names ........................................... i-iv Index - Common Names......................................... iv-vii Introduction ................................................................ viii Acknowledgements ................................................... viii Recent Graduates ........................................................ ix Trial Locations .............................................................. x Definitions of categories in report ............................. xi Summary report of graduates .............................. 1 - 77 Index – Botanical Name Abies balsamea ‘Nana’ .......................................... 1 Caragana roborovskyi ........................................... 7 Acer glabrum ......................................................... 1 Caragana tragacanthoides .................................... 7 Acer negundo ‘Sensation ...................................... 1 Celtis -

Price Increase: Quickship Temporarily Suspended
SURCHARGE: Effective for orders placed on and after July 15th, 2021, we will be adding a 2% surcharge to the order total, which will be reflected as a line item on your acknowledgment and invoice. The surcharge will end with the fall price increase and will not be applied to any future orders that receive the October 4th increase outlined below. PRICE INCREASE: Effective October 4th, 2021, we will implement a 5.5% price increase for all JSI products. Updated pricing will be available on e-tools following the release of the September DVD from 2020 Technologies and on the JSI website on October 4th. QUICKSHIP TEMPORARILY SUSPENDED: Effective immediately we are temporarily suspending our Quickship Program in order to deliver on our promise of on time shipments. We apologize for the inconvenience and appreciate your understanding. AMERICANA Americana Order Check List: Specify: □ 1. Model Number □ 2. Wood Species - Red Oak (RO) or Maple (M) □ 3. Wood Finish/Color □ 4. Upholstered seat style - horseshoe style available; add $20 list per chair/barstool; add $26 list per bench AMERICANA □ 5. Fabric Selection - vendor, pattern and color □ 6. Optional FRONT LEG casters - add $54 list; specify seat height NOT AVAILABLE on BARSTOOLS □ 7. Optional Trim Nails; add $101 list per chair/barstool; add $128 list per bench. Standard trim nail color is Antique Brass. Optional Black trim nails available. □ 8.Optional 1 ¾" Circle Back Cut Out - add $17 list per chair (select models) □ 9.Optional Rubber Cushion Nylon Glides - no upcharge Example: 301C RO HEN MOMENTUM VOX MYSTIC (model #) (wood species) (finish) (fabric vendor) (pattern name) (pattern color) 1. -

Cytogenetics of Fraxinus Mandshurica and F. Quadrangulata: Ploidy Determination and Rdna Analysis
Tree Genetics & Genomes (2020) 16:26 https://doi.org/10.1007/s11295-020-1418-6 ORIGINAL ARTICLE Cytogenetics of Fraxinus mandshurica and F. quadrangulata: ploidy determination and rDNA analysis Nurul Islam-Faridi1,2 & Mary E. Mason3 & Jennifer L. Koch4 & C. Dana Nelson5,6 Received: 22 July 2019 /Revised: 1 January 2020 /Accepted: 16 January 2020 # The Author(s) 2020 Abstract Ashes (Fraxinus spp.) are important hardwood tree species in rural, suburban, and urban forests of the eastern USA. Unfortunately, emerald ash borer (EAB, Agrilus planipennis) an invasive insect pest that was accidentally imported from Asia in the late 1980s–early 1990s is destroying them at an alarming rate. All North American ashes are highly susceptible to EAB, although blue ash (F. quadrangulata) may have some inherent attributes that provide it some protection. In contrast Manchurian ash (F. mandshurica) is relatively resistant to EAB having coevolved with the insect pest in its native range in Asia. Given its level of resistance, Manchurian ash has been considered for use in interspecies breeding programs designed to transfer resistance to susceptible North American ash species. One prerequisite for successful interspecies breeding is consistency in chromosome ploidy level and number between the candidate species. In the current study, we cytologically determined that both Manchurian ash and blue ash are diploids (2n) and have the same number of chromosomes (2n =2x = 46). We also characterized these species’ ribosomal gene families (45S and 5S rDNA) using fluorescence in situ hybridization (FISH). Both Manchurian and blue ash showed two 45S rDNA and one 5S rDNA sites, but blue ash appears to have an additional site of 45S rDNA. -

Yorkhill Green Spaces Wildlife Species List
Yorkhill Green Spaces Wildlife Species List April 2021 update Yorkhill Green Spaces Species list Draft list of animals, plants, fungi, mosses and lichens recorded from Yorkhill, Glasgow. Main sites: Yorkhill Park, Overnewton Park and Kelvinhaugh Park (AKA Cherry Park). Other recorded sites: bank of River Kelvin at Bunhouse Rd/ Old Dumbarton Rd, Clyde Expressway path, casual records from streets and gardens in Yorkhill. Species total: 711 Vertebrates: Amhibians:1, Birds: 57, Fish: 7, Mammals (wild): 15 Invertebrates: Amphipods: 1, Ants: 3, Bees: 26, Beetles: 21, Butterflies: 11, Caddisflies: 2, Centipedes: 3, Earthworms: 2, Earwig: 1, Flatworms: 1, Flies: 61, Grasshoppers: 1, Harvestmen: 2, Lacewings: 2, Mayflies: 2, Mites: 4, Millipedes: 3, Moths: 149, True bugs: 13, Slugs & snails: 21, Spiders: 14, Springtails: 2, Wasps: 13, Woodlice: 5 Plants: Flowering plants: 174, Ferns: 5, Grasses: 13, Horsetail: 1, Liverworts: 7, Mosses:17, Trees: 19 Fungi and lichens: Fungi: 24, Lichens: 10 Conservation Status: NameSBL - Scottish Biodiversity List Priority Species Birds of Conservation Concern - Red List, Amber List Last Common name Species Taxon Record Common toad Bufo bufo amphiban 2012 Australian landhopper Arcitalitrus dorrieni amphipod 2021 Black garden ant Lasius niger ant 2020 Red ant Myrmica rubra ant 2021 Red ant Myrmica ruginodis ant 2014 Buff-tailed bumblebee Bombus terrestris bee 2021 Garden bumblebee Bombus hortorum bee 2020 Tree bumblebee Bombus hypnorum bee 2021 Heath bumblebee Bombus jonellus bee 2020 Red-tailed bumblebee Bombus -

Manchurian Ash
Manchurian Ash III-101 Manchurian Ash Environmental Requirements (Fraxinus mandshurica) Soils Soil Texture - Adapted to a wide variety of soils. Soil pH - 5.5 to 8.0. General Description Windbreak Suitability Group - 1, 1K, 2, 2K, 3, 4, 4C 5. A medium to large tree similar to the native Black Ash in leaf characteristics. Has a slightly lower moisture Cold Hardiness requirement than black ash. Produces a very dense, oval to USDA Zone 3. rounded, shapely crown. One year twigs are golden colored. Lacy-textured foliage. The largest tree in North Water Dakota is 37 feet tall with a canopy spread of 25 feet. Prefers moist well-drained soils. Leaves and Buds Light Bud Arrangement - Opposite. Partial sun to full sun. Bud Color - Black or nearly so. Uses Bud Size - Terminal buds are ovate, pointed, 1/4 inch long. Leaf Type and Shape - Pinnate compound, 9 to 11 leaflets, Conservation/Windbreaks rachis slightly winged. Medium height tree for farmstead windbreaks. Leaf Margins - Sharply serrate. Wildlife Leaf Surface - Nearly smooth, usually pilose or hispid on the veins beneath, rufous-tomentose at leaflet bases. Seeds are eaten by some birds. Leaf Length - 10 to 12 inches; leaflets 3 to 5 inches. Agroforestry Products Leaf Width - 2 to 4 inches; leaflets 1 to 2 inches. Wood - Firewood, crafts. Leaf Color - Light green above; yellow fall color. Medicinal - Used for sores and itches. Flowers and Fruits Urban/Recreational Flower Type - Dioecious. Good landscape tree on moist sites or where additional Flower Color - Greenish-yellow. moisture can be supplied. Has a tailored, dense oval form, becoming more rounded with age. -

Invasive Plants Common in Connecticut
Invasive Plants Common in Connecticut Invasive Plants Common in Connecticut Norway Maple Scientific Name: Acer platanoides L. Origin: Europe & Asia Ecological Threat: Forms monotypic populations by dis- placing native trees, shrubs, and herbaceous understory plants. Once established, it creates a canopy of dense shade that prevents regeneration of native seedlings. Description/Biology: Plant: broad deciduous tree up to 90 ft. in height with broadly-rounded crown; bark is smooth at first but becomes black, ridged and furrowed with age. Leaves: paired, deciduous, dark green, pal- mate (like a hand), broader across than from base to tip, marginal teeth with long hair-like tips. Flowers, fruits and seeds: flowers in spring, bright yellow-green; fruits mature during summer into paired winged “samaras” joined broadly at nearly 180° angle; milky sap will ooze from cut veins or petiole. Similar Species: Other maples including sugar maple (Acer saccharum) and red maple (Acer rubrum). Distin- guish Norway by milky white sap, broad leaves, hair-like leaf tips, samara wings straight out, yellow fall foliage. Native Alternatives: Native maples like sugar maple (Acer saccharum) and red maple (Acer rubrum) Norway Maple Scientific Name: Acer platanoides L. Origin: Europe & Asia Ecological Threat: Forms monotypic populations by dis- placing native trees, shrubs, and herbaceous understory plants. Once established, it creates a canopy of dense shade that prevents regeneration of native seedlings. Description/Biology: Plant: broad deciduous tree up to 90 ft. in height with broadly-rounded crown; bark is smooth at first but becomes black, ridged and furrowed with age. Leaves: paired, deciduous, dark green, pal- mate (like a hand), broader across than from base to tip, marginal teeth with long hair-like tips. -

Wholesale Price List Fall 2010 - Spring 2011 Missmiss Jilene,Jilene, Hibiscushibiscus Plantplant Var.Var
HillisNursery Company, Inc. Wholesale Price List Fall 2010 - Spring 2011 MissMiss Jilene,Jilene, HibiscusHibiscus PlantPlant var.var. ‘Hillis‘Hillis Variegated’Variegated’ Office,Office, PackingPacking andand StorageStorage FacilitiesFacilities locatedlocated 44½½ milesmiles SouthSouth ofof McMinnvilleMcMinnville onon HighwayHighway 56.56. OnOn thethe cover:cover: WhiteWhite TrilliumsTrilliums Plant Index A G Q Alder ........................................................45 Ginkgo ......................................................54 Quince .....................................................17 Almond ....................................................28 Golden Chain Tree ................................56 R Althea ................................................21-23 Goldenrain Tree.....................................56 Raspberry ...............................................30 Apple..................................................78-79 Grape .................................................81-82 Redbud ............................................. 48-50 Ash .................................................... 53-54 H Redwood .................................................60 B Hackberry ...............................................48 Rose .........................................................30 Barberry ..................................................15 Hawthorn ..........................................52-53 S Bayberry .................................................27 Hazelnut ..................................................85 -

Eighth-Year Survival and Growth of Planted Replacement Tree Species in Black Ash ( Fraxinus Nigra ) Wetlands Threatened by Emera
Forest Ecology and Management 484 (2021) 118958 Contents lists available at ScienceDirect Forest Ecology and Management journal homepage: www.elsevier.com/locate/foreco Eighth-year survival and growth of planted replacement tree species in black ash (Fraxinus nigra) wetlands threatened by emerald ash borer in Minnesota, USA Brian J. Palik a,*, Anthony W. D’Amato b, Robert A. Slesak c, Doug Kastendick a, Chris Looney d, Josh Kragthorpe e a USDA Forest Service, Northern Research Station, Grand Rapids, MN 55744, USA b Rubenstein School of Environment and Natural Resources, University of Vermont, Burlington, VT 05405, USA c USDA Forest Service, Pacific Northwest Research Station, Olympia, WA 98512, USA d USDA Forest Service, Pacific Southwest Research Station, Davis, CA 95618, USA e Department of Forest Resources, University of Minnesota, St. Paul, MN 55108, USA ARTICLE INFO ABSTRACT Keywords: Black ash (Fraxinus nigra) is native to lowland forests of the western Great Lakes region, USA, where it often EAB comprises a majority of trees. Like all native ash in North America, black ash is threatened by emerald ash borer Foundational species (EAB; Agrilus planipennis), but the impacts from EAB mortality may be particularly severe in these forests given Tree regeneration the foundational role of black ash at regulating ecosystem function. Compounding the problem is that associated Silviculture tree species occur in low abundance and their abundance may be further reduced as habitat declines with climate Assisted migration Enrichment planting change. These converging threats point to the need for silvicultural intervention to establish replacement tree species in anticipation of EAB invasion. Here we report on a large-scale management experiment from Minne sota, USA that includes different silvicultural approaches for establishing replacement tree species in black ash forests.