Hymenoptera: Braconidae) from Iran
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Menu Samples
Menu Samples The Original Quick Six Rosemary Spiked Cannellini Crostini, Spaghetti al Cavolo Nero (Black Kale), Chicken with Herbs De Provence, Popcorn Cauliflower, Nori Crunch Salad with Avocado, Elana's Famous GuiltFree Cobbler The original MEAL that started the SPIEL. Six delicious, healthy and fast recipes including Elana's Famous Guiltfree Cobbler. You will be amazed at how easy it is to make good food and increase your popularity in your circle of friends!! This class is ideal for novices and cooks with more experience and will provide you with a 4 1/2 course dinner party menu (should you decide to accept the challenge), smaller dinner party menus and "quick fixes" just for you. A Quick Six: Hot Mediterranean Menu Roasted Tomato and Burrata Crostini, Pasta with Deconstructed Arugula Pesto, Edo's Mother's Swordfish, Shores of Capperi Potato Salad (no mayo), BiColore Salad, Strawberry Macedonia with Mint A tried and true menu that will transport you to the hotblooded flavors of the southern Mediterranean. (Note, the food is not spicy, just good.) The Quick Six has become one of the signature classes of Meal and a Spiel. Come learn six fast, easy, healthy and delicious recipes that will make you popular amongst your circle of friends. This class is ideal for novices and cooks with more experience and will provide you with a 4 1/2 course dinner party menu (should you decide to accept the challenge), smaller dinner party menus and "quick fixes" just for you. Hot Tuscan Nights Tomato and Basil Bruschetta, Sliced Steak with Arugula, Shaved Parmigiano and a Balsamic Reduction, BakedNotFried Little Potato Sticks with Rosemary and Thyme, Greek Yogurt Panna Cotta with Rosemary Grove Peaches The warm windy Italian countryside sets a perfect tone for a romantic dinner amongst lovers and friends. -

Revision of the Indian Microplitis Foerster (Hymenoptera: Braconidae: Microgastrinae), with Description of One New Species
Zootaxa 3620 (3): 429–452 ISSN 1175-5326 (print edition) www.mapress.com/zootaxa/ Article ZOOTAXA Copyright © 2013 Magnolia Press ISSN 1175-5334 (online edition) http://dx.doi.org/10.11646/zootaxa.3620.3.5 http://zoobank.org/urn:lsid:zoobank.org:pub:1B04F939-9FFA-4B01-B851-7A6A7EDDF131 Revision of the Indian Microplitis Foerster (Hymenoptera: Braconidae: Microgastrinae), with description of one new species ANKITA GUPTA National Bureau of Agriculturally Important Insects, Post Bag No. 2491, H. A. Farm Post, Bellary Road, Hebbal, Bangalore 560 024, Karnataka, India. E-mail: [email protected] Abstract The Indian fauna of the genus Microplitis Foerster, 1862 is revised. An illustrated key to eight species including the description of one new species, M. murkyi sp. nov., is provided. Six previously described species, namely: M. ajmerensis Rao & Kurian, M. demolitor Wilkinson, M. indicus Marsh, M. manilae Ashmead, M. prodeniae Rao & Kurian, and M. spodopterae Rao & Kurian are elaborated with taxonomical variations and extended distribution. Two species, M. bageshri Sathe, Inamdar & Dawale and M. dipika (Bhatnagar) are considered incertae sedis. A new combination is suggested for Snellenius maculipennis (Szepligeti) which is placed into synonymy with Microplitis. Information on taxonomic history of the genus, diagnostic characters of all the included species, distribution and host relationships are provided. Key words: Parasitic wasps, host relationships, India, Microplitis, Microgastrinae, revision Introduction The apomorphic genus Microplitis was established by Foerster in 1862 with the type species Microgaster sordipes Nees von Esenbeck. The species are larval endoparasitoids of agriculturally important pests, particularly lepidopteran species of Helicoverpa and Spodoptera. The hosts mostly belong to the family Noctuidae, and to some extent Sphingidae and Lymantriidae, athough some other families have also been recorded. -

ALAY in Villa Dining Menu
1 IN VILLA DINING To ensure that you experience the very best of Anantara Layan Phuket Resort, we have create ‘In Villa’ service combining a variety of cuisines from each of our unique restaurant menus, with dining experiences that are gracious, comforting and truly memorable. Start your day decadently, with a choice from our extensive breakfast menu. Choices range from a healthy ‘Vitality’ selection to a more exotic Asian breakfast, all delivered to the privacy of your villa terrace whilst you wake slowly to a Phuket sunrise. Our supremely talented culinary team, have carefully chosen a menu to suit all tastes, but should there be anything that we have missed, we are more than happy to accommodate your request where possible. Dining By Design Page 2 – 13 Breakfast: 6.00 am - 11.00 am Page 14 – 18 All Day Dining: 11.00 am - 10.30 pm Page 19 - 24 Children’s Menu Page 25 - 27 TV Snacks Page 28 Night Menu: 10.30 pm - 6.00 am Page 29 - 31 Vegetarian & Vegan menu Page 31 Beverage Page 32 - 39 For enquiries and all in villa dining orders, please dial “At Your Service”. Prices are in Thai Baht and are subject to 10% service charge and applicable government tax Vegan Vegetarian Spicy Dishes Contains Pork Consuming raw or undercooked meats, poultry, seafood, shellfish, or eggs may increase your risk of food borne illness. Please inquire with senior management if you have any dietary restrictions, allergies or special considerations. 2 DINING BY DESIGN We hope you are enjoying the warm tropical weather of Phuket, our wonderful resort and friendly hospitality so renowned in Thailand. -

NEW DEPUTY COMMANDER QUARANTINE! HOW to Cope?
SHAPE Community Life April 2020 SHAPE WELCOMES NORTH MACEDONIA NEW DEPUTY COMMANDER QUARANTINE! HOW TO COPe? EDITOR’s Letter Dear Shapians, Winds of change are blowing globally throughout our society in the last months, and they are changing our mindset and values. All media channels try to reinforce positivity instead of negativity, trying to get light from darkness, putting away any blues. Social networks are encouraging us: read books, watch films, cook more, talk with family, play board games, listen to music... There comes a time when the mind becomes saturated and we have to put a defense mechanism in place. Some pictures represent the ephemeral of our society, depicting our cities like in a post-apocalyptic film. But, like in any war, the key thing is to maintain a morale of victory, to remain calm and to be sure that, all together, we will be able to win. Our business code has to adapt to the new landscape: working from home, meetings online, duty travel reductions, etc. We will have to make sacrifices, we will have to adapt to new habits and new ways to interrelate with others, with family, friends, colleagues... And the most important thing: in the end, we will be better people, we will pay more attention to things we thought were not important, and, above all, we will win. Stay safe! By the way, a 30th NATO member, North Macedonia, has joined us in March 2020. Welcome! María José Tezanos Bustamante Community Event & Communication Management SHAPE Morale & Welfare Branch SHAPE Community Life (SCL) is an authorized unofficial magazine, Officer-in-Charge published monthly by Base Support Group (BSG). -

Cooking Light 2001 Annual Recipe Index
1 Cooking Light 2001 Annual Recipe Index APPETIZERS Lemon-Poppy Seed Muffins, Mar 208 Artichoke-Crab Dip with Cumin-Dusted Pita Chips, J/F 83 Low-Fat Italian-Style Bread, June 160 Asian Party Mix, Dec 92 Mama’s Corn Bread, Nov 212 Asian Peanut Dip, Apr 117 Mandarin Pancakes, J/F 106 Bagna Cauda, Sept 110 Multigrain Bread, J/F 134 Baked Beef Empanadas, Sept 138 Olive Bread, J/F 81 Baked Egg Rolls, Mar 154 Orange Bubble Bread, J/F 130 Baked Italian Oysters, Nov 115 Oven-Puffed Pancake, Mar 123 Black Bean Dip with Tortilla Chips, June 97 Paraguayan Corn Bread, Sept 134 Creamy Oysters Rockefeller Dip, Nov 116 Parmesan, Garlic, and Basil Twists, Dec 112 Cumin-Dusted Pita Chips, J/F 83 Poppy Seed and Onion Crescent Rolls, Dec 112 Curried Crab Cakes, Dec 89 Poppy Seed Twists, Nov 196 Deviled Eggs, Aug 166 Potato Fougasse, Dec 114 Eggplant Spread with Yogurt and Parsley, July 148 Pumpkin-Cinnamon Streusel Buns, Oct 182 Endive Stuffed with Goat Cheese and Walnuts, Sept 102 Pumpkin-Date Loaf with Cream Cheese Swirl, Dec 112 Flamed Chorizo (Chorizo a la Llama), Aug 136 Pumpkin Waffles, Oct 181 Grilled Stuffed Portobello Mushrooms, June 166 Raisin-Rosemary Rye Bread, Oct 124 Layered Bean Dip, Sept 118 Speedy Focaccia with Fennel and Thyme, Mar 134 Layered Chili, Cheese, and Roasted-Corn Dip, Mar 205 Spiced Fig and Walnut Bread, Oct 123 Marinated Mushrooms, Apr 214 Spinach Corn Bread with Mango Salsa, J/F 148 Meat, Bulgur, and Rice Dolmades, July 142 Strawberry-and-Cream Cheese-Filled Muffins, Mar 206 Mexican Bean Dip, July 171 Sweet Potato Bread -

GLOBAL GOURMET About Theimportant Workthisorganization Isaccomplishingacross Thestate
GLOBAL GOURMET Bring the world closer with enticing menus from all corners. GOURMET GLOBAL FRENCH FÊTE DEMO SIP + SAVOR Michael Diem // Members $60, Nonmembers $65 (21+, ID Required) Escape to Provence by way of the PCC Cooks classroom. The arrival of fresh summer produce makes the perfect backdrop for learning new culinary techniques like braising a chicken, crafting a perfectly fluffed soufflé and pairing dishes with elegant French wines. This demo covers both savory and sweet — and of course, cheese. MENU: Chicken Provençal on Sautéed Greens Chèvre Souffle Raspberry Frangipane Tart French wines to pair Dietary Notes: With poultry, dairy and eggs. SUMMER IN THE MEDITERRANEAN HANDS-ON SIP + SAVOR Abby Canfield // Members $60, Nonmembers $65 (21+, ID Required) Sun-ripened ingredients shine bright in Mediterranean cuisine, and with a handful of simple techniques, you will learn to create four flavorful dishes from scratch. Get hands-on practice transforming seasonal produce and take home new skills, like making flatbread and grilling lamb, that you can adapt throughout the year. MENU: Flatbread with Za’atar, Local Labneh and Grilled and Fresh Heirloom Tomatoes Smashed Cucumber Salad with Preserved Lemon, Mint and Chiles Herbed Lamb Kebab with Lemon-Tahini Sauce Cardamom-stewed Stone Fruit with Honeyed Yogurt and Pistachios Wines to pair Dietary Notes: With meat, dairy and eggs. PERUVIAN CHICKEN DINNER DEMO Jennifer Reyes // Members $50, Nonmembers $55 Ever wanted to learn how to spatchcock a chicken? (Don’t even know what spatchcocking is?) This class is for you! Jennifer will demonstrate this technique designed to yield a perfect roast chicken while you discover the secrets of unique and flavorful Peruvian marinades and sauces. -
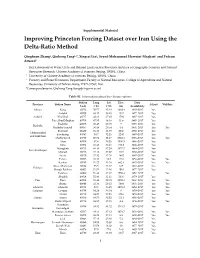
Improving Princeton Forcing Dataset Over Iran Using the Delta-Ratio Method
Supplemental Material Improving Princeton Forcing Dataset over Iran Using the Delta-Ratio Method Qinghuan Zhang1, Qiuhong Tang1,2*, Xingcai Liu1, Seyed-Mohammad Hosseini-Moghari1 and Pedram Attarod3 1 Key Laboratory of Water Cycle and Related Land Surface Processes, Institute of Geographic Sciences and Natural Resources Research, Chinese Academy of Sciences, Beijing, 100101, China 2 University of Chinese Academy of Sciences, Beijing, 100101, China 3 Forestry and Forest Economics Department, Faculty of Natural Resources, College of Agriculture and Natural Resources, University of Tehran, Karaj, 77871-31587, Iran *Correspondence to: Qiuhong Tang ([email protected]) Table S1. Information about the climate stations. Station Long Lat Elev. Data Province Station Name Adjust Validate Code (° E) (° N) (m) Availability Alborz Karaj 40752 50.57 35.48 1292.9 1985–2017 Yes Ardebil 40708 48.17 38.15 1332 1977–2017 Yes Ardebil Khalkhal 40717 48.31 37.38 1796 1987–2017 Yes Pars Abad Moghan 40700 47.55 39.39 31.9 1985–2017 Yes Bushehr 40858 50.49 28.58 9 1986–2017 Yes Bushehr Bushehr Coastal 40857 50.49 28.54 8.4 1951–2017 Yes Yes Boroojen 99459 51.18 31.59 2260 1988–2017 Yes Chaharmahal Koohrang 40797 50.7 32.26 2285 1987–2017 Yes and Bakhtiari Shahre Kord 40798 50.51 32.17 2048.9 1956–2017 Yes Yes Ahar 40704 47.4 38.26 1390.5 1986–2017 Yes Jolfa 40702 45.40 38.45 736.2 1986–2017 Yes Maragheh 40713 46.16 37.24 1477.7 1984–2017 Yes East Azarbaijan Mianeh 40716 47.42 37.27 1110 1987–2017 Yes Sarab 40710 47.32 37.56 1682 1987–2017 Yes Tabriz 40706 46.17 -

A Review of Unusual Species of Cotesia (Hymenoptera, Braconidae
A peer-reviewed open-access journal ZooKeys 580:A 29–44review (2016) of unusual species of Cotesia (Hymenoptera, Braconidae, Microgastrinae)... 29 doi: 10.3897/zookeys.580.8090 RESEARCH ARTICLE http://zookeys.pensoft.net Launched to accelerate biodiversity research A review of unusual species of Cotesia (Hymenoptera, Braconidae, Microgastrinae) with the first tergite narrowing at midlength Ankita Gupta1, Mark Shaw2, Sophie Cardinal3, Jose Fernandez-Triana3 1 ICAR-National Bureau of Agricultural Insect Resources, P. B. No. 2491, H. A. Farm Post, Bellary Road, Hebbal, Bangalore,560 024, India 2 National Museums of Scotland, Edinburgh, United Kingdom 3 Canadian National Collection of Insects, Ottawa, Canada Corresponding author: Ankita Gupta ([email protected]) Academic editor: K. van Achterberg | Received 9 February 2016 | Accepted 14 March 2016 | Published 12 April 2016 http://zoobank.org/9EBC59EC-3361-4DD0-A5A1-D563B2DE2DF9 Citation: Gupta A, Shaw M, Cardinal S, Fernandez-Triana J (2016) A review of unusual species of Cotesia (Hymenoptera, Braconidae, Microgastrinae) with the first tergite narrowing at midlength. ZooKeys 580: 29–44.doi: 10.3897/zookeys.580.8090 Abstract The unusual species ofCotesia (Hymenoptera, Braconidae, Microgastrinae) with the first tergite narrow- ing at midlength are reviewed. One new species, Cotesia trabalae sp. n. is described from India and com- pared with Cotesia pistrinariae (Wilkinson) from Africa, the only other species sharing the same character of all the described species worldwide. The generic -

Oligocene-Miocene Ramp System (Asmari Formation) in the NW of the Zagros Basin, Iran: Microfacies, Paleoenvironment and Depositional Sequence
56 Vaziri-MoghaddamRevista Mexicana et al. de Ciencias Geológicas, v. 27, núm. 1, 2010, p. 56-71 Oligocene-Miocene ramp system (Asmari Formation) in the NW of the Zagros basin, Iran: Microfacies, paleoenvironment and depositional sequence Hossein Vaziri-Moghaddam1,*, Ali Seyrafian1, Azizolah Taheri2, and Homayoon Motiei3 1 Department of Geology, Faculty of Sciences, University of Isfahan, Isfahan, Iran, 81746-73441. 2 Geology Department, Faculty of Earth Science, Shahrood University of Technology, Shahroud, Iran. 3 National Iranian Oil Company Research and Development Division, Tehran, Iran. * [email protected] ABSTRACT The Asmari Formation deposited in the Zagros foreland basin during the Oligocene-Miocene. Four different measured sections were studied in this area in order to interpret the facies, depositional environment and sequence stratigraphy of the Asmari Formation. In this study, thirteen different microfacies types have been recognized, which can be grouped into six depositional environments: tidal flat, restricted lagoon, open lagoon, shoal, slope and basin. The Asmari Formation represents sedimentation on a carbonate ramp. Four third-order sequences are identified, on the basis of deepening and shallowing patterns in the microfacies and the distribution of the Oligocene-Miocene foraminifers. The depositional sequences 1, 2 and 3 were observed in Dehluran and Kabirkuh-Darrehshahr areas, and are synchronous with a period of either erosion or non-deposition represented by unconformities in Mamulan and Sepid Dasht areas. Key words: microfacies, paleoenvironment, ramp, Asmari Formation, Zagros basin, Iran. RESUMEN La Formación Asmari se depositó en el antepaís de la cuenca Zagros durante el Oligoceno-Mioceno. Se estudiaron y midieron cuatro secciones diferentes en esta área para interpretar las facies, ambiente de depósito y la secuencia estratigráfica de la Formación Asmari. -

1590-1601 Issn 2322-5149 ©2014 Jnas
Journal of Novel Applied Sciences Available online at www.jnasci.org ©2014 JNAS Journal-2014-3-S2/1590-1601 ISSN 2322-5149 ©2014 JNAS Trend analysis of the changes in urban hierarchy of Khuzestan: a sustainable development perspective Mohammad Ajza Shokouhi1* and Jawad Bawi2 1- Associate Professor of Geography and Urban Planning at Ferdowsi University of Mashhad 2- PhD student in Geography and Urban Planning, International Branch of Ferdowsi University of Mashhad Corresponding author: Mohammad Ajza Shokouhi ABSTRACT: This paper deals with the changes in the urban hierarchy of Khuzestan during a period of 50 years (1956-2006) determining the extent of changes in urbanization and the potential spatial differences between the cities in this province from the perspective of sustainable development. Adopting a descriptive-analytic approach and employing various models such as tensile modulus, primate city indicators, urban concentration index (three-city and four-city), the rank-size rule, the present paper analyzes the factors influencing the urban networks in Khuzestan. It follows from the results of the study that the urban networks of the province, have been heavily affected by developments so that Abadan which used to have the first rank in Khuzestan has lost its rank to Ahwaz due to the administrative, political, and commercial centrality of Ahwaz. The imposed war (of Iraq against Iran) has also caused abrupt changes in the population and urban hierarchy. Therefore, urban networks of Khuzestan influenced by factors such as immigration do not have a spatial balance (and hence stability) currently. Interestingly, the results suggest that the spatial distance between the first city Ahwaz with other cities is growing exponentially. -

Final Programme
Final Programme Follow us OFFICIAL CORPORATE SUPPORTER @IBAevents #IBARome Expert and professional advice since 1975 The law firm Studio Legale Tributario Fantozzi & Associati was established in 1975 by Augusto Fantozzi, a lawyer and full professor of tax law at the ‘’La Sapienza’’ and ‘’LUISS’’ Universities in Rome. Professor Fantozzi was the Italian Minister for Finance and the Minister of Foreign Trade between 1995 and 1998, and he is a member of the Board of Directors and the Board of Statutory Auditors of several leading Italian companies and multinational corporations. The Firm has offices in Rome, Milan and Bologna. With 8 Senior Partners, all lawyers or chartered accountants, and more than 30 legal professionals, the Firm is highly specialised in tax law, and as such provides clients with advice on Italian and international fiscal law, and assists them in tax litigation. Thanks to the years of experience of its partners and legal professionals, the Firm can offer clients full support in resolving tax and corporate issues, both nationally and internationally. Over the years the Firm has dealt with the fiscal aspects of numerous important corporate and financial operations carried out by public and private companies, banks, finance companies and insurance undertakings, and has become their go-to adviser on ordinary and extraordinary tax matters. ROMA | MILANO | BOLOGNA www.fantozzieassociati.com Follow us CONTENTS Contents @IBAevents #IBARome Introduction by the President of the IBA 5 IBA Management Board and IBA Staff 6 Opening -

Thank You So Much for Choosing to Dine with Us. We Are Incredibly
Thank you so much for choosing to dine with us. We are incredibly grateful to have this opportunity to serve you again! What To Know Before Dining ❖ We ask you to provide a primary covid contact on your reservation prior to dining with us. For walk-ins, please be prepared to provide a contact to the host. All parties will be required to provide at least one contact person’s full name, full address, mobile number and email address. ❖ We ask that you limit your dining time to 1 hour for Brunch and 1.5 hours for Dinner on busier services to allow guests to enjoy dining out again. ❖ We ask you to please be patient and kind to our staff and to one another as we all try to adjust to a new normal. ❖ We will seat you as close to your reservation time as possible. ❖ We ask that you arrive complete with your party to avoid crowding and allow for safe dining space. ❖ We ask that you arrive on time as we will only hold reservations for up to 15 minutes. After 15 minutes, your reservation will be placed on the waitlist for the next available table. ❖ In the event of inclement weather, we unfortunately cannot permit guests to come inside, therefore please plan accordingly. We would be happy to reschedule your reservation or you may place a takeout or delivery order by visiting our website. What To Expect When You Arrive At Maison Pickle ❖ Body Temperature check is required upon arrival and guests will be stamped to acknowledge that it has been done.