CRITICAL APPRAISAL of the SCIENTIFIC LITERATURE: Sorting out the Good, the Bad, and the Ugly
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Descriptive Statistics (Part 2): Interpreting Study Results
Statistical Notes II Descriptive statistics (Part 2): Interpreting study results A Cook and A Sheikh he previous paper in this series looked at ‘baseline’. Investigations of treatment effects can be descriptive statistics, showing how to use and made in similar fashion by comparisons of disease T interpret fundamental measures such as the probability in treated and untreated patients. mean and standard deviation. Here we continue with descriptive statistics, looking at measures more The relative risk (RR), also sometimes known as specific to medical research. We start by defining the risk ratio, compares the risk of exposed and risk and odds, the two basic measures of disease unexposed subjects, while the odds ratio (OR) probability. Then we show how the effect of a disease compares odds. A relative risk or odds ratio greater risk factor, or a treatment, can be measured using the than one indicates an exposure to be harmful, while relative risk or the odds ratio. Finally we discuss the a value less than one indicates a protective effect. ‘number needed to treat’, a measure derived from the RR = 1.2 means exposed people are 20% more likely relative risk, which has gained popularity because of to be diseased, RR = 1.4 means 40% more likely. its clinical usefulness. Examples from the literature OR = 1.2 means that the odds of disease is 20% higher are used to illustrate important concepts. in exposed people. RISK AND ODDS Among workers at factory two (‘exposed’ workers) The probability of an individual becoming diseased the risk is 13 / 116 = 0.11, compared to an ‘unexposed’ is the risk. -

EBM Notebook Numbers Needed to Treat Derived from Meta-Analysis: a Word of Caution
Evid Based Med: first published as 10.1136/ebm.8.2.36 on 1 March 2003. Downloaded from EBM notebook Numbers needed to treat derived from meta-analysis: a word of caution In clinical trials, treatment effects from binary outcomes, such the NNT will be applied, and information on the time horizon as “alive” or “dead”, can be presented in various ways (eg, rela- of the study (eg, the median follow up time). tive risk reduction [RRR] and absolute risk reduction We illustrate these points using the meta-analysis of [ARR]).1–2 (See glossary for definitions and calculations). Alter- randomised controlled trials investigating the effect of n-3 poly- natively, the number needed to treat (NNT) is an expression of the unsaturated fatty acids on clinical endpoints in patients with number of patients who need to be treated to prevent one coronary heart disease as an example.11–12 The meta-analysis additional adverse event.2–4 Mathematically, the NNT equals the found a 19% (CI 10% to 27%) RRR of overall mortality favour- reciprocal of the ARR. Many journals now report results from ing the use of n-3 polyunsaturated fatty acids. In the clinical trials using the NNT, along with 95% confidence inter- Evidence-Based Medicine structured summary of this meta- vals (CIs).5 analysis, the NNT derived from a pooled risk difference, and 11 Since its introduction,3 a debate has ensued whether based on an average baseline risk of 9.5%, is 73 (CI 49 to 147). reporting NNTs from meta-analyses is misleading.467 ACP However, this estimate does not reflect the high variability of Journal Club and Evidence-Based Medicine have devoted attention baseline risk of the included trials. -
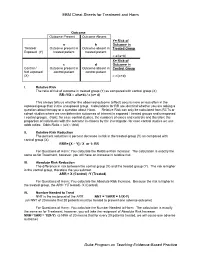
EBM Cheat Sheets for Treatment and Harm Duke Program on Teaching
EBM Cheat Sheets for Treatment and Harm Outcome Outcome Present Outcome Absent Y= Risk of a b Outcome in Treated/ Outcome present in Outcome absent in Treated Group Exposed (Y) treated patient treated patient = a/(a+b) X= Risk of c d Outcome in Control / Outcome present in Outcome absent in Control Group Not exposed control patient control patient (X) = c/(c+d) I. Relative Risk The ratio of risk of outcome in treated group (Y) as compared with control group (X) RR=Y/X = a/(a+b) / c (c+ d) This always tells us whether the observed outcome (effect) occurs more or less often in the exposed group than in the unexposed group. Calculations for RR are identical whether you are asking a question about therapy or a question about Harm. Relative Risk can only be calculated from RCTs or cohort studies where we can determine outcomes of interest in exposed / treated groups and unexposed / control groups. (Note: for case control studies, the numbers of cases and controls and therefore the proportion of individuals with the outcome is chosen by the investigator- for case control studies we use odds ratios: Odds Ratio = (a/c) / (b/d) II. Relative Risk Reduction The percent reduction is percent decrease in risk in the treated group (Y) as compared with control group (X) RRR= [X – Y] / X or 1- RR For Questions of Harm: You calculate the Relative Risk Increase: The calculation is exactly the same as for Treatment, however, you will have an increase in relative risk. III. Absolute Risk Reduction The difference in risk between the control group (X) and the treated group (Y). -

Outcome Reporting Bias in COVID-19 Mrna Vaccine Clinical Trials
medicina Perspective Outcome Reporting Bias in COVID-19 mRNA Vaccine Clinical Trials Ronald B. Brown School of Public Health and Health Systems, University of Waterloo, Waterloo, ON N2L3G1, Canada; [email protected] Abstract: Relative risk reduction and absolute risk reduction measures in the evaluation of clinical trial data are poorly understood by health professionals and the public. The absence of reported absolute risk reduction in COVID-19 vaccine clinical trials can lead to outcome reporting bias that affects the interpretation of vaccine efficacy. The present article uses clinical epidemiologic tools to critically appraise reports of efficacy in Pfzier/BioNTech and Moderna COVID-19 mRNA vaccine clinical trials. Based on data reported by the manufacturer for Pfzier/BioNTech vaccine BNT162b2, this critical appraisal shows: relative risk reduction, 95.1%; 95% CI, 90.0% to 97.6%; p = 0.016; absolute risk reduction, 0.7%; 95% CI, 0.59% to 0.83%; p < 0.000. For the Moderna vaccine mRNA-1273, the appraisal shows: relative risk reduction, 94.1%; 95% CI, 89.1% to 96.8%; p = 0.004; absolute risk reduction, 1.1%; 95% CI, 0.97% to 1.32%; p < 0.000. Unreported absolute risk reduction measures of 0.7% and 1.1% for the Pfzier/BioNTech and Moderna vaccines, respectively, are very much lower than the reported relative risk reduction measures. Reporting absolute risk reduction measures is essential to prevent outcome reporting bias in evaluation of COVID-19 vaccine efficacy. Keywords: mRNA vaccine; COVID-19 vaccine; vaccine efficacy; relative risk reduction; absolute risk reduction; number needed to vaccinate; outcome reporting bias; clinical epidemiology; critical appraisal; evidence-based medicine Citation: Brown, R.B. -

Clinical Epidemiologic Definations
CLINICAL EPIDEMIOLOGIC DEFINATIONS 1. STUDY DESIGN Case-series: Report of a number of cases of disease. Cross-sectional study: Study design, concurrently measure outcome (disease) and risk factor. Compare proportion of diseased group with risk factor, with proportion of non-diseased group with risk factor. Case-control study: Retrospective comparison of exposures of persons with disease (cases) with those of persons without the disease (controls) (see Retrospective study). Retrospective study: Study design in which cases where individuals who had an outcome event in question are collected and analyzed after the outcomes have occurred (see also Case-control study). Cohort study: Follow-up of exposed and non-exposed defined groups, with a comparison of disease rates during the time covered. Prospective study: Study design where one or more groups (cohorts) of individuals who have not yet had the outcome event in question are monitored for the number of such events, which occur over time. Randomized controlled trial: Study design where treatments, interventions, or enrollment into different study groups are assigned by random allocation rather than by conscious decisions of clinicians or patients. If the sample size is large enough, this study design avoids problems of bias and confounding variables by assuring that both known and unknown determinants of outcome are evenly distributed between treatment and control groups. Bias (Syn: systematic error): Deviation of results or inferences from the truth, or processes leading to such deviation. See also Referral Bias, Selection Bias. Recall bias: Systematic error due to the differences in accuracy or completeness of recall to memory of past events or experiences. -

Enrichment Strategies for Clinical Trials to Support Determination of Effectiveness of Human Drugs and Biological Products Guidance for Industry
Enrichment Strategies for Clinical Trials to Support Determination of Effectiveness of Human Drugs and Biological Products Guidance for Industry U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER) Center for Biologics Evaluation and Research (CBER) March 2019 Clinical/Medical Enrichment Strategies for Clinical Trials to Support Determination of Effectiveness of Human Drugs and Biological Products Guidance for Industry Additional copies are available from: Office of Communications, Division of Drug Information Center for Drug Evaluation and Research Food and Drug Administration 10001 New Hampshire Ave., Hillandale Bldg., 4th Floor Silver Spring, MD 20993-0002 Phone: 855-543-3784 or 301-796-3400; Fax: 301-431-6353; Email: [email protected] https://www.fda.gov/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/default.htm and/or Office of Communication, Outreach, and Development Center for Biologics Evaluation and Research Food and Drug Administration 10903 New Hampshire Ave., Bldg. 71, rm. 3128 Silver Spring, MD 20993-0002 Phone: 800-835-4709 or 240-402-8010; Email: [email protected] https://www.fda.gov/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/Guidances/default.htm U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER) Center for Biologics Evaluation and Research (CBER) March 2019 Clinical/Medical TABLE OF CONTENTS I. INTRODUCTION............................................................................................................ -

NUMBER NEEDED to TREAT (CONTINUATION) Confidence
Number Needed to treat Confidence Interval for NNT • As with other estimates, it is important that the uncertainty in the estimated number needed to treat is accompanied by a confidence interval. • A common method is that proposed by Cook and Sacket (1995) and based on calculating first the confidence interval NUMBER NEEDED TO TREAT for the difference between the two proportions. • The result of this calculation is 'inverted' (ie: 1/CI) to give a (CONTINUATION) confidence interval for the NNT. Hamisu Salihu, MD, PhD Confidence Interval for NNT Confidence Interval for NNT • This computation assumes that ARR estimates from a sample of similar trials are normally distributed, so we can use the 95th percentile point from the standard normal distribution, z0.95 = 1.96, to identify the upper and lower boundaries of the 95% CI. Solution Class Exercise • In a parallel clinical trial to test the benefit of additional anti‐ hyperlipidemic agent to standard protocol, patients with acute Myocardial Infarction (MI) were randomly assigned to the standard therapy or the new treatment that contains standard therapy and simvastatin (an HMG‐CoA reductase inhibitor). At the end of the study, of the 2223 patients in the control group 622 died as compared to 431 of 2221 patients in the Simvastatin group. – Calculate the NNT and the 95% CI for Simvastatin – Is Simvastatin beneficial as additional therapy in acute cases of MI? 1 Confidence Interval Number Needed to for NNT Treat • The NNT to prevent one additional death is 12 (95% • A negative NNT indicates that the treatment has a CI, 9‐16). -

Evidence-Based Clinical Question Does Dantrolene Sodium Prevent Recurrent Exertional Rhabdomyolysis in Horses? M
EQUINE VETERINARY EDUCATION / AE / March 2007 97 Evidence-based Clinical Question Does dantrolene sodium prevent recurrent exertional rhabdomyolysis in horses? M. A. HOLMES University of Cambridge, Department of Veterinary Medicine, Madingley Road, Cambridge CB3 0ES, UK. Three part question of any other clinical manifestation of disease. An arbitrary level of >900 iu/l was selected as a case definition to provide In Thoroughbred horses (population) does treatment with a means of counting the number of animals that might be dantrolene sodium (intervention) reduce the recurrence of described as constituting a case of exertional rhabdomyolysis exertional rhabdomyolysis (outcome)? (ER). Clinical cases of ER may exhibit considerably greater levels of CK and it should not be implied that lower CK levels Clinical scenario may not be associated with clinical signs of ER. The key results are presented as absolute risk reductions A client requests advice on the prevention of recurrent (ARR) and relative risk reductions (RRR). The ARR indicates the exertional rhabdomyloysis (RER) for a Thoroughbred horse proportion of all horses in the treatment group that have an with a history of this condition. The veterinarian is aware that improved clinical outcome as a result of the treatment (i.e. the dantrolene sodium has been suggested as a suitable proportion of horses that benefit from dantrolene treatment). preventive treatment. Both the trials described here are unusual in that a number of horses are being treated that might not be considered Search strategy appropriate candidates for prophylactic treatment. There were 74 horses in the Edwards trial and 3 horses in the McKenzie Pubmed/Medline (1966–Jan 2007) (http://pubmed.org/): trial that failed to develop high CK levels either with or dantrolene AND equine. -

Understanding Measures of Treatment Effect in Clinical Trials a K Akobeng
54 Arch Dis Child: first published as 10.1136/adc.2004.061747 on 21 December 2004. Downloaded from CURRENT TOPIC Understanding measures of treatment effect in clinical trials A K Akobeng ............................................................................................................................... Arch Dis Child 2005;90:54–56. doi: 10.1136/adc.2004.052233 Evidence based medicine implies that healthcare admitted with RSV infection was10.6% for those receiving placebo and 4.8% for those receiving professionals are expected to base their practice on the prophylactic palivizumab. It should be noted that best available evidence. This means that we should acquire AR and all the other measures of treatment effect the necessary skills for appraising the medical literature, discussed below are statistical estimates and the uncertainty in the estimates should be accom- including the ability to understand and interpret the results panied by confidence intervals. of published articles. This article discusses in a simple, practical, ‘non-statistician’ fashion some of the important Absolute risk reduction outcome measures used to report clinical trials comparing In a study comparing a group of patients who different treatments or interventions. Absolute and relative were exposed to a particular intervention with another group who did not receive the interven- risk measures are explained, and their merits and demerits tion, the absolute risk reduction (ARR) is discussed. The article aims to encourage healthcare calculated as the arithmetic difference in the professionals to appreciate the use and misuse of these AR of an outcome in individuals who were outcome measures and to empower them to calculate these exposed to the intervention and the AR of the outcome in those unexposed to the intervention. -

A Number-Needed-To-Treat Analysis of the EMAX Trial
Efficacy of Umeclidinium/Vilanterol Versus Umeclidinium or Salmeterol: A Number-Needed-to-Treat Analysis of the EMAX Trial Poster No. 1091 (A3326) 1 2 3 1 2 3 4 5 Respiratory Medicine and Allergology, Lund University, Lund, Sweden; Centre de Pneumologie, Institut Universitaire de Cardiologie et de Pneumologie de Québec, Université Laval, QC, Canada; Department of Medicine, Pulmonary and Critical Care Medicine, University Medical Center Giessen and Marburg, Bjermer L , Maltais F , Vogelmeier CF , Naya I , Jones PW , 4 5 6 6 5 5 7 8 9 Philipps-Universität Marburg, Germany, Member of the German Center for Lung Research (DZL); Global Specialty & Primary Care, GSK, Brentford, Middlesex, UK (at the time of the study) and RAMAX Ltd., Bramhall, Cheshire, UK; GSK, Brentford, Middlesex, UK; Precise Approach Ltd, contingent worker on assignment at Tombs L , Boucot I , Compton C , Lipson DA , Keeley T , Kerwin E GSK, Stockley Park West, Uxbridge, Middlesex, UK; 7Respiratory Clinical Sciences, GSK, Collegeville, PA, USA and Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA, USA; 8GSK, Stockley Park West, Uxbridge, Middlesex, UK; 9Clinical Research Institute of Southern Oregon, Medford, OR, USA Background Results ● The relative treatment benefits with long-acting muscarinic antagonist/long-acting Patients β -agonist (LAMA/LABA) combination therapy versus LAMA or LABA monotherapy have not been Table 1. Patient demographics and clinical characteristics Figure 2. Hazard ratio for a first moderate/severe exacerbation or CID at Week 24 2 ● Demographics and baseline characteristics are shown in Table 1. demonstrated in symptomatic, inhaled corticosteroid (ICS)-free patients with COPD who have a low UMEC/VI vs UMEC UMEC/VI vs SAL UMEC/VI UMEC SAL risk of exacerbations. -

SUPPLEMENTARY APPENDIX Powering Bias and Clinically
SUPPLEMENTARY APPENDIX Powering bias and clinically important treatment effects in randomized trials of critical illness Darryl Abrams, MD, Sydney B. Montesi, MD, Sarah K. L. Moore, MD, Daniel K. Manson, MD, Kaitlin M. Klipper, MD, Meredith A. Case, MD, MBE, Daniel Brodie, MD, Jeremy R. Beitler, MD, MPH Supplementary Appendix 1 Table of Contents Content Page 1. Additional Methods 3 2. Additional Results 4 3. Supplemental Tables a. Table S1. Sample size by trial type 6 b. Table S2. Accuracy of control group mortality by trial type 6 c. Table S3. Predicted absolute risk reduction in mortality by trial type 6 d. Table S4. Difference in predicted verses observed treatment effect, absolute risk difference 6 e. Table S5. Characteristics of trials by government funding 6 f. Table S6. Proportion of trials for which effect estimate includes specified treatment effect size, 7 all trials g. Table S7. Inconclusiveness of important effect size for reduction in mortality among trials 7 without a statistically significant treatment benefit h. Table S8. Inconclusiveness of important effect size for increase in mortality among trials 7 without a statistically significant treatment harm 4. Supplemental Figures a. Figure S1. Screening and inclusion of potentially eligible studies 8 b. Figure S2. Butterfly plot of predicted and observed mortality risk difference, grouped by 9 journal c. Figure S3. Butterfly plot of predicted and observed mortality risk difference, grouped by 10 disease area d. Figure S4. Trial results according to clinically important difference in mortality on absolute 11 and relative scales, grouped by journal e. Figure S5. Trial results according to clinically important difference in mortality on absolute 12 and relative scales, grouped by disease area f. -

Routine Circumcision of Newborn Infants Re
A Trade-off Analysis of Routine Newborn Circumcision Dimitri A. Christakis, MD, MPH*‡; Eric Harvey, PharmD, MBA‡; Danielle M. Zerr, MD, MPH*; Chris Feudtner, MD, PhD*§; Jeffrey A. Wright, MD*; and Frederick A. Connell, MD, MPH‡i Abstract. Background. The risks associated with outine circumcision of newborn infants re- newborn circumcision have not been as extensively mains controversial.1–3 Although a recent evaluated as the benefits. Rpolicy statement by the American Academy Objectives. The goals of this study were threefold: 1) of Pediatrics recommended that parents be given to derive a population-based complication rate for new- accurate and unbiased information regarding the born circumcision; 2) to calculate the number needed to risks and benefits of the procedure,3 accomplishing harm for newborn circumcision based on this rate; and this task of well informed consent is hindered both 3) to establish trade-offs based on our complication rates and published estimates of the benefits of circumcision by the lack of precise information about potential including the prevention of urinary tract infections and harm and by the lack of clear ways to present this penile cancer. information. Methods. Using the Comprehensive Hospital Ab- The benefits of circumcision have been described stract Reporting System for Washington State, we retro- in numerous previous studies using a variety of spectively examined routine newborn circumcisions methodologies.4–8 Reported benefits include reduc- performed over 9 years (1987–1996). We used Interna- tion in the risk of penile cancer,9 urinary tract in- tional Classification of Diseases, Ninth Revision codes fections (UTIs),4,5 and sexually transmitted diseas- to identify both circumcisions and complications and es.10,11 Although the extent to which circumcision limited our analyses to children without other surgical decreases the risk of each of these outcomes has procedures performed during their initial birth hospi- been debated,6,12–15 a consensus appears to be talization.