Final Report Conservation Genetics of Spring
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Endangered Species Act 2018
▪ Requires regulators to consider potential effects on T&E species during permitting process ▪ Must evaluate whether they are present ▪ If present, will there be any effects? ▪ Each plant or animal type has particular set of rules about when protective measures need to be placed in permit ▪ Terrestrial species typically only require protections when present within footprint of activity or within a buffer zone of habitat features (roost trees, hibernacula, etc.) ▪ Aquatic species require protections if project is within a certain distance upstream and/or if the project disturbs an upstream drainage area greater than a given size Species Scientific Name Eastern cougar Felis concolor cougar* Indiana bat Myotis sodalis Virginia big-eared bat Corynorhinus townsendii virginianus Northern long-eared bat Myotis septentrionalis Cheat Mountain salamander Plethodon nettingi Diamond darter Crystallaria cincotta Madison Cave isopod Antrolana lira Species Scientific Name Clubshell mussel Pleurobema clava Fanshell mussel Cyprogenia stegaria James spiny mussel Pleurobema collina Pink mucket mussel Lampsilis abrupta Rayed bean mussel Villosa fabalis Sheepnose mussel Plethobasus cyphyus Spectaclecase mussel Cumberlandia monodonta Species Scientific Name Snuffbox mussel Epioblasma triquetra Tubercled blossom pearly mussel Epioblasma torulosa torulosa Guyandotte River crayfish Cambarus veteranus Big Sandy crayfish Canbarus callainus Flat-spired three toothed land snail Triodopsis platysayoides Harperella Ptilimnium nodosum Northeastern bulrush Scirpus ancistrochaetus -

Research Funding (Total $2,552,481) $15,000 2019
CURRICULUM VITAE TENNESSEE AQUARIUM CONSERVATION INSTITUTE 175 BAYLOR SCHOOL RD CHATTANOOGA, TN 37405 RESEARCH FUNDING (TOTAL $2,552,481) $15,000 2019. Global Wildlife Conservation. Rediscovering the critically endangered Syr-Darya Shovelnose Sturgeon. $10,000 2019. Tennessee Wildlife Resources Agency. Propagation of the Common Logperch as a host for endangered mussel larvae. $8,420 2019. Tennessee Wildlife Resources Agency. Monitoring for the Laurel Dace. $4,417 2019. Tennessee Wildlife Resources Agency. Examining interactions between Laurel Dace (Chrosomus saylori) and sunfish $12,670 2019. Trout Unlimited. Southern Appalachian Brook Trout propagation for reintroduction to Shell Creek. $106,851 2019. Private Donation. Microplastic accumulation in fishes of the southeast. $1,471. 2019. AZFA-Clark Waldram Conservation Grant. Mayfly propagation for captive propagation programs. $20,000. 2019. Tennessee Valley Authority. Assessment of genetic diversity within Blotchside Logperch. $25,000. 2019. Riverview Foundation. Launching Hidden Rivers in the Southeast. $11,170. 2018. Trout Unlimited. Propagation of Southern Appalachian Brook Trout for Supplemental Reintroduction. $1,471. 2018. AZFA Clark Waldram Conservation Grant. Climate Change Impacts on Headwater Stream Vertebrates in Southeastern United States $1,000. 2018. Hamilton County Health Department. Step 1 Teaching Garden Grants for Sequoyah School Garden. $41,000. 2018. Riverview Foundation. River Teachers: Workshops for Educators. $1,000. 2018. Tennessee Valley Authority. Youth Freshwater Summit $20,000. 2017. Tennessee Valley Authority. Lake Sturgeon Propagation. $7,500 2017. Trout Unlimited. Brook Trout Propagation. $24,783. 2017. Tennessee Wildlife Resource Agency. Assessment of Percina macrocephala and Etheostoma cinereum populations within the Duck River Basin. $35,000. 2017. U.S. Fish and Wildlife Service. Status surveys for conservation status of Ashy (Etheostoma cinereum) and Redlips (Etheostoma maydeni) Darters. -

Indiana Species April 2007
Fishes of Indiana April 2007 The Wildlife Diversity Section (WDS) is responsible for the conservation and management of over 750 species of nongame and endangered wildlife. The list of Indiana's species was compiled by WDS biologists based on accepted taxonomic standards. The list will be periodically reviewed and updated. References used for scientific names are included at the bottom of this list. ORDER FAMILY GENUS SPECIES COMMON NAME STATUS* CLASS CEPHALASPIDOMORPHI Petromyzontiformes Petromyzontidae Ichthyomyzon bdellium Ohio lamprey lampreys Ichthyomyzon castaneus chestnut lamprey Ichthyomyzon fossor northern brook lamprey SE Ichthyomyzon unicuspis silver lamprey Lampetra aepyptera least brook lamprey Lampetra appendix American brook lamprey Petromyzon marinus sea lamprey X CLASS ACTINOPTERYGII Acipenseriformes Acipenseridae Acipenser fulvescens lake sturgeon SE sturgeons Scaphirhynchus platorynchus shovelnose sturgeon Polyodontidae Polyodon spathula paddlefish paddlefishes Lepisosteiformes Lepisosteidae Lepisosteus oculatus spotted gar gars Lepisosteus osseus longnose gar Lepisosteus platostomus shortnose gar Amiiformes Amiidae Amia calva bowfin bowfins Hiodonotiformes Hiodontidae Hiodon alosoides goldeye mooneyes Hiodon tergisus mooneye Anguilliformes Anguillidae Anguilla rostrata American eel freshwater eels Clupeiformes Clupeidae Alosa chrysochloris skipjack herring herrings Alosa pseudoharengus alewife X Dorosoma cepedianum gizzard shad Dorosoma petenense threadfin shad Cypriniformes Cyprinidae Campostoma anomalum central stoneroller -

A Thesis Entitled Molecular, Morphological, and Biogeographic Resolution of Cryptic Taxa in the Greenside Darter Etheostoma Blen
A Thesis Entitled Molecular, morphological, and biogeographic resolution of cryptic taxa in the Greenside Darter Etheostoma blennioides complex By Amanda E. Haponski Submitted as partial fulfillment of the requirements for The Master of Science Degree in Biology (Ecology-track) ____________________________ Advisor: Dr. Carol A. Stepien ____________________________ Committee Member: Dr. Timothy G. Fisher ____________________________ Committee Member: Dr. Johan F. Gottgens ____________________________ College of Graduate Studies The University of Toledo December 2007 Copyright © 2007 This document is copyrighted material. Under copyright law, no parts of this document may be reproduced without the expressed permission of the author. An Abstract of Molecular, morphological, and biogeographic resolution of cryptic taxa in the Greenside Darter Etheostoma blennioides complex Amanda E. Haponski Submitted as partial fulfillment of the requirements for The Master of Science Degree in Biology (Ecology-track) The University of Toledo December 2007 DNA sequencing has led to the resolution of many cryptic taxa, which are especially prevalent in the North American darter fishes (Family Percidae). The Greenside Darter Etheostoma blennioides commonly occurs in the lower Great Lakes region, where two putative subspecies, the eastern “Allegheny” type E. b. blennioides and the western “Prairie” type E. b. pholidotum , overlap. The objective of this study was to test the systematic identity and genetic divergence distinguishing the two subspecies in areas of sympatry and allopatry in comparison to other subspecies and close relatives. DNA sequences from the mtDNA cytochrome b gene and control region and the nuclear S7 intron 1 comprising a total of 1,497 bp were compared from 294 individuals across 18 locations, including the Lake Erie basin and the Allegheny, Meramec, Obey, Ohio, Rockcastle, Susquehanna, and Wabash River systems. -
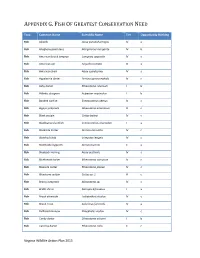
Fish of Greatest Conservation Need
APPENDIX G. FISH OF GREATEST CONSERVATION NEED Taxa Common Name Scientific Name Tier Opportunity Ranking Fish Alewife Alosa pseudoharengus IV a Fish Allegheny pearl dace Margariscus margarita IV b Fish American brook lamprey Lampetra appendix IV c Fish American eel Anguilla rostrata III a Fish American shad Alosa sapidissima IV a Fish Appalachia darter Percina gymnocephala IV c Fish Ashy darter Etheostoma cinereum I b Fish Atlantic sturgeon Acipenser oxyrinchus I b Fish Banded sunfish Enneacanthus obesus IV c Fish Bigeye jumprock Moxostoma ariommum III c Fish Black sculpin Cottus baileyi IV c Fish Blackbanded sunfish Enneacanthus chaetodon I a Fish Blackside darter Percina maculata IV c Fish Blotched chub Erimystax insignis IV c Fish Blotchside logperch Percina burtoni II a Fish Blueback Herring Alosa aestivalis IV a Fish Bluebreast darter Etheostoma camurum IV c Fish Blueside darter Etheostoma jessiae IV c Fish Bluestone sculpin Cottus sp. 1 III c Fish Brassy Jumprock Moxostoma sp. IV c Fish Bridle shiner Notropis bifrenatus I a Fish Brook silverside Labidesthes sicculus IV c Fish Brook Trout Salvelinus fontinalis IV a Fish Bullhead minnow Pimephales vigilax IV c Fish Candy darter Etheostoma osburni I b Fish Carolina darter Etheostoma collis II c Virginia Wildlife Action Plan 2015 APPENDIX G. FISH OF GREATEST CONSERVATION NEED Fish Carolina fantail darter Etheostoma brevispinum IV c Fish Channel darter Percina copelandi III c Fish Clinch dace Chrosomus sp. cf. saylori I a Fish Clinch sculpin Cottus sp. 4 III c Fish Dusky darter Percina sciera IV c Fish Duskytail darter Etheostoma percnurum I a Fish Emerald shiner Notropis atherinoides IV c Fish Fatlips minnow Phenacobius crassilabrum II c Fish Freshwater drum Aplodinotus grunniens III c Fish Golden Darter Etheostoma denoncourti II b Fish Greenfin darter Etheostoma chlorobranchium I b Fish Highback chub Hybopsis hypsinotus IV c Fish Highfin Shiner Notropis altipinnis IV c Fish Holston sculpin Cottus sp. -

Endangered Species
FEATURE: ENDANGERED SPECIES Conservation Status of Imperiled North American Freshwater and Diadromous Fishes ABSTRACT: This is the third compilation of imperiled (i.e., endangered, threatened, vulnerable) plus extinct freshwater and diadromous fishes of North America prepared by the American Fisheries Society’s Endangered Species Committee. Since the last revision in 1989, imperilment of inland fishes has increased substantially. This list includes 700 extant taxa representing 133 genera and 36 families, a 92% increase over the 364 listed in 1989. The increase reflects the addition of distinct populations, previously non-imperiled fishes, and recently described or discovered taxa. Approximately 39% of described fish species of the continent are imperiled. There are 230 vulnerable, 190 threatened, and 280 endangered extant taxa, and 61 taxa presumed extinct or extirpated from nature. Of those that were imperiled in 1989, most (89%) are the same or worse in conservation status; only 6% have improved in status, and 5% were delisted for various reasons. Habitat degradation and nonindigenous species are the main threats to at-risk fishes, many of which are restricted to small ranges. Documenting the diversity and status of rare fishes is a critical step in identifying and implementing appropriate actions necessary for their protection and management. Howard L. Jelks, Frank McCormick, Stephen J. Walsh, Joseph S. Nelson, Noel M. Burkhead, Steven P. Platania, Salvador Contreras-Balderas, Brady A. Porter, Edmundo Díaz-Pardo, Claude B. Renaud, Dean A. Hendrickson, Juan Jacobo Schmitter-Soto, John Lyons, Eric B. Taylor, and Nicholas E. Mandrak, Melvin L. Warren, Jr. Jelks, Walsh, and Burkhead are research McCormick is a biologist with the biologists with the U.S. -

ECOLOGY of NORTH AMERICAN FRESHWATER FISHES
ECOLOGY of NORTH AMERICAN FRESHWATER FISHES Tables STEPHEN T. ROSS University of California Press Berkeley Los Angeles London © 2013 by The Regents of the University of California ISBN 978-0-520-24945-5 uucp-ross-book-color.indbcp-ross-book-color.indb 1 44/5/13/5/13 88:34:34 AAMM uucp-ross-book-color.indbcp-ross-book-color.indb 2 44/5/13/5/13 88:34:34 AAMM TABLE 1.1 Families Composing 95% of North American Freshwater Fish Species Ranked by the Number of Native Species Number Cumulative Family of species percent Cyprinidae 297 28 Percidae 186 45 Catostomidae 71 51 Poeciliidae 69 58 Ictaluridae 46 62 Goodeidae 45 66 Atherinopsidae 39 70 Salmonidae 38 74 Cyprinodontidae 35 77 Fundulidae 34 80 Centrarchidae 31 83 Cottidae 30 86 Petromyzontidae 21 88 Cichlidae 16 89 Clupeidae 10 90 Eleotridae 10 91 Acipenseridae 8 92 Osmeridae 6 92 Elassomatidae 6 93 Gobiidae 6 93 Amblyopsidae 6 94 Pimelodidae 6 94 Gasterosteidae 5 95 source: Compiled primarily from Mayden (1992), Nelson et al. (2004), and Miller and Norris (2005). uucp-ross-book-color.indbcp-ross-book-color.indb 3 44/5/13/5/13 88:34:34 AAMM TABLE 3.1 Biogeographic Relationships of Species from a Sample of Fishes from the Ouachita River, Arkansas, at the Confl uence with the Little Missouri River (Ross, pers. observ.) Origin/ Pre- Pleistocene Taxa distribution Source Highland Stoneroller, Campostoma spadiceum 2 Mayden 1987a; Blum et al. 2008; Cashner et al. 2010 Blacktail Shiner, Cyprinella venusta 3 Mayden 1987a Steelcolor Shiner, Cyprinella whipplei 1 Mayden 1987a Redfi n Shiner, Lythrurus umbratilis 4 Mayden 1987a Bigeye Shiner, Notropis boops 1 Wiley and Mayden 1985; Mayden 1987a Bullhead Minnow, Pimephales vigilax 4 Mayden 1987a Mountain Madtom, Noturus eleutherus 2a Mayden 1985, 1987a Creole Darter, Etheostoma collettei 2a Mayden 1985 Orangebelly Darter, Etheostoma radiosum 2a Page 1983; Mayden 1985, 1987a Speckled Darter, Etheostoma stigmaeum 3 Page 1983; Simon 1997 Redspot Darter, Etheostoma artesiae 3 Mayden 1985; Piller et al. -

Aquatic Fish Report
Aquatic Fish Report Acipenser fulvescens Lake St urgeon Class: Actinopterygii Order: Acipenseriformes Family: Acipenseridae Priority Score: 27 out of 100 Population Trend: Unknown Gobal Rank: G3G4 — Vulnerable (uncertain rank) State Rank: S2 — Imperiled in Arkansas Distribution Occurrence Records Ecoregions where the species occurs: Ozark Highlands Boston Mountains Ouachita Mountains Arkansas Valley South Central Plains Mississippi Alluvial Plain Mississippi Valley Loess Plains Acipenser fulvescens Lake Sturgeon 362 Aquatic Fish Report Ecobasins Mississippi River Alluvial Plain - Arkansas River Mississippi River Alluvial Plain - St. Francis River Mississippi River Alluvial Plain - White River Mississippi River Alluvial Plain (Lake Chicot) - Mississippi River Habitats Weight Natural Littoral: - Large Suitable Natural Pool: - Medium - Large Optimal Natural Shoal: - Medium - Large Obligate Problems Faced Threat: Biological alteration Source: Commercial harvest Threat: Biological alteration Source: Exotic species Threat: Biological alteration Source: Incidental take Threat: Habitat destruction Source: Channel alteration Threat: Hydrological alteration Source: Dam Data Gaps/Research Needs Continue to track incidental catches. Conservation Actions Importance Category Restore fish passage in dammed rivers. High Habitat Restoration/Improvement Restrict commercial harvest (Mississippi River High Population Management closed to harvest). Monitoring Strategies Monitor population distribution and abundance in large river faunal surveys in cooperation -

Part IV: Scoring Criteria for the Index of Biotic Integrity to Monitor
Part IV: Scoring Criteria for the Index of Biotic Integrity to Monitor Fish Communities in Wadeable Streams in the Coosa and Tennessee Drainage Basins of the Ridge and Valley Ecoregion of Georgia Georgia Department of Natural Resources Wildlife Resources Division Fisheries Management Section 2020 Table of Contents Introduction………………………………………………………………… ……... Pg. 1 Map of Ridge and Valley Ecoregion………………………………..……............... Pg. 3 Table 1. State Listed Fish in the Ridge and Valley Ecoregion……………………. Pg. 4 Table 2. IBI Metrics and Scoring Criteria………………………………………….Pg. 5 References………………………………………………….. ………………………Pg. 7 Appendix 1…………………………………………………………………. ………Pg. 8 Coosa Basin Group (ACT) MSR Graphs..………………………………….Pg. 9 Tennessee Basin Group (TEN) MSR Graphs……………………………….Pg. 17 Ridge and Valley Ecoregion Fish List………………………………………Pg. 25 i Introduction The Ridge and Valley ecoregion is one of the six Level III ecoregions found in Georgia (Part 1, Figure 1). It is drained by two major river basins, the Coosa and the Tennessee, in the northwestern corner of Georgia. The Ridge and Valley ecoregion covers nearly 3,000 square miles (United States Census Bureau 2000) and includes all or portions of 10 counties (Figure 1), bordering the Piedmont ecoregion to the south and the Blue Ridge ecoregion to the east. A small portion of the Southwestern Appalachians ecoregion is located in the upper northwestern corner of the Ridge and Valley ecoregion. The biotic index developed by the GAWRD is based on Level III ecoregion delineations (Griffith et al. 2001). The metrics and scoring criteria adapted to the Ridge and Valley ecoregion were developed from biomonitoring samples collected in the two major river basins that drain the Ridge and Valley ecoregion, the Coosa (ACT) and the Tennessee (TEN). -

Warmwater Streams Report 2012
FISHERIES REPORT Warmwater Streams and Rivers Tennessee Wildlife Resources Agency--Region IV Report 13-02 2012 FISHERIES REPORT REPORT NO. 13-02 WARMWATER STREAM FISHERIES REPORT REGION IV 2012 Prepared by Bart D. Carter Rick D. Bivens Carl E. Williams and James W. Habera TENNESSEE WILDLIFE RESOURCES AGENCY ii Development of this report was financed in part by funds from Federal Aid in Fish and Wildlife Restoration (TWRA Project 4350) (Public Law 91-503) as documented in Federal Aid Project FW-6. This program receives Federal Aid in Fish and Wildlife Restoration. Under Title VI of the Civil Rights Act of 1964 and Section 504 of the Interior prohibits discrimination on the basis of race, color, national origin, or handicap. If you believe you have been discriminated against in any program, activity, or facility as described above, or if you desire further information, please write to: Office of Equal Opportunity, U.S. Department of the Interior, Washington D.C. 20240. Cover: Cumberland darter (Etheostoma susanae) collected from Capuchin Creek, Campbell County. iii TABLE OF CONTENTS Page INTRODUCTION 1 METHODS 2 Tennessee River System: Little River 9 Pellissippi Parkway Extension Surveys 18 Holston River 27 French Broad River 38 Clinch River System: Cove Creek 47 French Broad River System: Pigeon River 50 Cumberland River System: Capuchin Creek 56 New River System: Straight Fork 59 Jake Branch 61 Clear Fork Cumberland River System: Hudson Branch 63 Terry Creek 65 Lick Fork 67 Little Elk Creek 69 Stinking Creek 72 Louse Creek 75 SUMMARY 78 LITERATURE CITED 81 iv INTRODUCTION The fish fauna of Tennessee is the most diverse in the United States, with approximately 307 species of native fish and about 30 to 33 introduced species (Etnier and Starnes 1993). -

Candy Darter Recovery Outline
U.S. Fish & Wildlife Service Candy Darter Recovery Outline Photo by Corey Dunn, University of Missouri Species Name: Candy Darter (Etheostoma osburni) Species Range: Upper Kanawha River Basin including the Gauley, Greenbrier, and New River Watersheds including portions of Greenbrier, Pocahontas, Nicholas, and Webster counties in West Virginia; and Bland, Giles, and Wythe counties, Virginia. The range of the species is shown in figure 1 below. Recovery Priority Number: 5; explanation provided below Listing Status: Endangered; November 21, 2019; 83 FR 58747 Lead Regional Office/Cooperating RO(s): Northeast Region, Hadley MA Lead Field Office/Cooperating FO(s): West Virginia Field Office, Elkins WV; Southwestern Virginia Field Office, Abingdon, VA; White Sulphur Springs National Fish Hatchery, White Sulphur Springs, WV Lead Contact: Barbara Douglas, 304-636-6586 ext 19. [email protected] 1) Background This section provides a brief overview of the ecology and conservation of the candy darter. This information is more fully described in the Species Status Assessment (SSA), the final listing rule, and the proposed critical habitat rule. These documents are available at: https://www.fws.gov/northeast/candydarter/ 1 Type and Quality of Available Information to Date: Important Information Gaps and Treatment of Uncertainties One of the primary threats that resulted in the listing of the candy darter under the Endangered Species Act (ESA) is the spread of the introduced variegate darter (Etheostoma variatum). This species hybridizes with the candy darter. Key information gaps and areas of uncertainty for the recovery and management of the candy darter include whether there are any habitat or other natural factors that could limit the spread of variegate darter. -

OCCASIONAL PAPERS of the MUSEUM of ZOOLOGY UNIVERSITY of MICHIGAN Asx ARBOR,MICHIGAN
OCCASIONAL PAPERS OF THE MUSEUM OF ZOOLOGY UNIVERSITY OF MICHIGAN Asx ARBOR,MICHIGAN ETHEOSTOAPA ACUTICEPS, .A NET41 DARTER FROM THE TENNESSEE RIVER SYSTEM, TWITH REMARKS ON THE SUBGENUS NOTHOATOTUS PRIOR10 the impoundment of the South Fork of thc I-Iolston River, Tennessee, a qualitative fish survey oT the area to be inundated was conducted by a party representing the Tennessee Valley Authority, the Tennessee Department of Conservation, and The University of Michigan. On the last day of field operations, September, 1947, two specimens of an undescribed darter were secured. In an attempt to obtain additional material the locality was revisited in June, 1949, by Gerald P. Cooper, John D. Kilby, and myself. Low water made success possible, because we found that the species was apparently confined to the deeper, faster riffles near the center of the stream. The darter was so rare that only four individuals were seined in three hours. The South Holston Reservoir is now full and the only known locality for Etheostomn acuticeps lies beneath 190 feet of water. Since it is unlikely that this swift-water species can tolerate quiet water, it is hoped that other stations of occurrence may be disco~.ered.To my companions in the field work, the late R. W. Eschmeyer, then with the Tennessee Valley Authority, Jack Chance, formerly with the Tennessee Department of Conservation, Gerald P. Cooper, Michigan Department of Conservation, and John D. Kilby, University of Flor- ida, I am sincerely grateful. For several years I have been assembling data leading toward a revision of the bluebreast darters (subgenus Nothonotus).