Elevated Levels of OH Observed in Haze Events During Wintertime in Central Beijing
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Music Globally Protected Marks List (GPML) Music Brands & Music Artists
Music Globally Protected Marks List (GPML) Music Brands & Music Artists © 2012 - DotMusic Limited (.MUSIC™). All Rights Reserved. DotMusic reserves the right to modify this Document .This Document cannot be distributed, modified or reproduced in whole or in part without the prior expressed permission of DotMusic. 1 Disclaimer: This GPML Document is subject to change. Only artists exceeding 1 million units in sales of global digital and physical units are eligible for inclusion in the GPML. Brands are eligible if they are globally-recognized and have been mentioned in established music trade publications. Please provide DotMusic with evidence that such criteria is met at [email protected] if you would like your artist name of brand name to be included in the DotMusic GPML. GLOBALLY PROTECTED MARKS LIST (GPML) - MUSIC ARTISTS DOTMUSIC (.MUSIC) ? and the Mysterians 10 Years 10,000 Maniacs © 2012 - DotMusic Limited (.MUSIC™). All Rights Reserved. DotMusic reserves the right to modify this Document .This Document 10cc can not be distributed, modified or reproduced in whole or in part 12 Stones without the prior expressed permission of DotMusic. Visit 13th Floor Elevators www.music.us 1910 Fruitgum Co. 2 Unlimited Disclaimer: This GPML Document is subject to change. Only artists exceeding 1 million units in sales of global digital and physical units are eligible for inclusion in the GPML. 3 Doors Down Brands are eligible if they are globally-recognized and have been mentioned in 30 Seconds to Mars established music trade publications. Please -

The Top 7000+ Pop Songs of All-Time 1900-2017
The Top 7000+ Pop Songs of All-Time 1900-2017 Researched, compiled, and calculated by Lance Mangham Contents • Sources • The Top 100 of All-Time • The Top 100 of Each Year (2017-1956) • The Top 50 of 1955 • The Top 40 of 1954 • The Top 20 of Each Year (1953-1930) • The Top 10 of Each Year (1929-1900) SOURCES FOR YEARLY RANKINGS iHeart Radio Top 50 2018 AT 40 (Vince revision) 1989-1970 Billboard AC 2018 Record World/Music Vendor Billboard Adult Pop Songs 2018 (Barry Kowal) 1981-1955 AT 40 (Barry Kowal) 2018-2009 WABC 1981-1961 Hits 1 2018-2017 Randy Price (Billboard/Cashbox) 1979-1970 Billboard Pop Songs 2018-2008 Ranking the 70s 1979-1970 Billboard Radio Songs 2018-2006 Record World 1979-1970 Mediabase Hot AC 2018-2006 Billboard Top 40 (Barry Kowal) 1969-1955 Mediabase AC 2018-2006 Ranking the 60s 1969-1960 Pop Radio Top 20 HAC 2018-2005 Great American Songbook 1969-1968, Mediabase Top 40 2018-2000 1961-1940 American Top 40 2018-1998 The Elvis Era 1963-1956 Rock On The Net 2018-1980 Gilbert & Theroux 1963-1956 Pop Radio Top 20 2018-1941 Hit Parade 1955-1954 Mediabase Powerplay 2017-2016 Billboard Disc Jockey 1953-1950, Apple Top Selling Songs 2017-2016 1948-1947 Mediabase Big Picture 2017-2015 Billboard Jukebox 1953-1949 Radio & Records (Barry Kowal) 2008-1974 Billboard Sales 1953-1946 TSort 2008-1900 Cashbox (Barry Kowal) 1953-1945 Radio & Records CHR/T40/Pop 2007-2001, Hit Parade (Barry Kowal) 1953-1935 1995-1974 Billboard Disc Jockey (BK) 1949, Radio & Records Hot AC 2005-1996 1946-1945 Radio & Records AC 2005-1996 Billboard Jukebox -
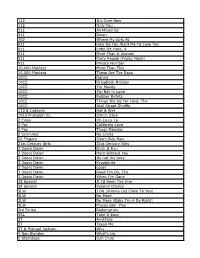
112 It's Over Now 112 Only You 311 All Mixed up 311 Down
112 It's Over Now 112 Only You 311 All Mixed Up 311 Down 702 Where My Girls At 911 How Do You Want Me To Love You 911 Little Bit More, A 911 More Than A Woman 911 Party People (Friday Night) 911 Private Number 10,000 Maniacs More Than This 10,000 Maniacs These Are The Days 10CC Donna 10CC Dreadlock Holiday 10CC I'm Mandy 10CC I'm Not In Love 10CC Rubber Bullets 10CC Things We Do For Love, The 10CC Wall Street Shuffle 112 & Ludacris Hot & Wet 1910 Fruitgum Co. Simon Says 2 Evisa Oh La La La 2 Pac California Love 2 Pac Thugz Mansion 2 Unlimited No Limits 20 Fingers Short Dick Man 21st Century Girls 21st Century Girls 3 Doors Down Duck & Run 3 Doors Down Here Without You 3 Doors Down Its not my time 3 Doors Down Kryptonite 3 Doors Down Loser 3 Doors Down Road I'm On, The 3 Doors Down When I'm Gone 38 Special If I'd Been The One 38 Special Second Chance 3LW I Do (Wanna Get Close To You) 3LW No More 3LW No More (Baby I'm A Do Right) 3LW Playas Gon' Play 3rd Strike Redemption 3SL Take It Easy 3T Anything 3T Tease Me 3T & Michael Jackson Why 4 Non Blondes What's Up 5 Stairsteps Ooh Child 50 Cent Disco Inferno 50 Cent If I Can't 50 Cent In Da Club 50 Cent In Da Club 50 Cent P.I.M.P. (Radio Version) 50 Cent Wanksta 50 Cent & Eminem Patiently Waiting 50 Cent & Nate Dogg 21 Questions 5th Dimension Aquarius_Let the sunshine inB 5th Dimension One less Bell to answer 5th Dimension Stoned Soul Picnic 5th Dimension Up Up & Away 5th Dimension Wedding Blue Bells 5th Dimension, The Last Night I Didn't Get To Sleep At All 69 Boys Tootsie Roll 8 Stops 7 Question -

Catalogmusic873 919.Pdf
INDEX 874 INDEX TITLE PAGE NO. TITLE PAGE NO. TITLE PAGE NO. A Acoustic Guitar Solo Fingerstyle Basics ..........707, 761 Afro-Cuban Slap Bass Lines............................693, 812 Acoustic Guitar Tab White Pages ....................394, 792 Aguilera, Christina – Stripped ..................................73 Aaberg, Philip – Piano Solos....................................72 Acoustic Instrumentalists – Guitar One Air Supply – Best of .................................................73 Above All – 15 Classic Praise Songs...............422, 535 Presents.....................................................394, 798 Aladdin – Big-Note Piano.......................................571 Above All – 50 EZ Inspirational Favorites...............422 INDEX Acoustic Piano Ballads...................................395, 545 Aladdin – Music Manuscript Paper ........................854 Above All Else, Your Name Be Praised!...................500 Acoustic Rock – Fingerstyle Guitar.........................395 Aladdin – P/V/G .....................................................271 Abrams, Daniel – Put Your Hands on the Acoustic Rock – Guitar Play-Along Volume 18.......789 Aladdin – Piano Fun! .............................................297 Piano and Play!..................................................848 Acoustic Rock – Guitar Recorded Versions............395 Aladdin – Recorder Fun!........................................294 Accompaniment Basics – Acoustic Guitar ......707, 741 Acoustic Rock – Guitar Technique Series.......733, 742 Aladdin – Xylotone -

Preprint. Discussion Started: 6 May 2020 C Author(S) 2020
https://doi.org/10.5194/acp-2020-362 Preprint. Discussion started: 6 May 2020 c Author(s) 2020. CC BY 4.0 License. Elevated levels of OH observed in haze events during wintertime in central Beijing Eloise J. Slater1, Lisa K. Whalley1,2, Robert Woodward-Massey1,a, Chunxiang Ye1,a, James D Lee3,4, Freya Squires4, James R. Hopkins3,4, Rachel E Dunmore4, Marvin Shaw3,4, Jacqueline F. Hamilton4, Alastair C 3,4 5,b 5 5 5 6 6 6 Lewis , Leigh R. Crilley , Louisa Kramer , William Bloss , Tuan Vu , Yele Sun , Weiqi Xu , Siyao Yue , 5 Lujie Ren6, W. Joe F. Acton7, C. Nicholas Hewitt7, Xinming Wang8, Pingqing Fu9 and Dwayne E. Heard1 1School of Chemistry, University of Leeds, Leeds, LS2 9JT, UK 2National Centre for Atmospheric Science, University of Leeds, Leeds, LS2 9JT, UK 3National Centre for Atmospheric Science, University of York, Heslington, York, YO10 5DD, UK 4Wolfson Atmospheric Chemistry Laboratories, Department of Chemistry, University of York, Heslington, York, 10 YO10 5DD, UK 5School of Geography, Earth and Environmental Sciences, University of Birmingham, B15 2TT, Birmingham, UK 6State Key Laboratory of Atmospheric Boundary Layer Physics and Atmospheric Chemistry, Institute for Atmospheric Physics, Chinese Academy of Sciences, 40 Huayanli, Chaoyang District, Beijing 100029, China 7Lancaster Environment Centre, Lancaster University, Lancaster, LA1 4YW, UK 15 8State Key Laboratory of Organic Geochemistry, Guangzhou Institute of Geochemistry, Chinese Academy of Sciences, 511 Kehua Street, Wushan, Tianhe District, Guangzhou, GD 510640 , China -

New Radicals
new radicals 30th March 2012 UTZON ROOM SYDNEY OPERA HOUSE 1st April 2012 THE STREET THEATRE CANBERRA ENSEMBLE OFFSPRING new radicals Artistic Directors Claire Edwardes & Damien Ricketson program Matthew Wright: Breakdown flute, clarinet, percussion, keyboard, violin & cello [AP] Michael Norris: Save Yourself flute, clarinet, accordion, synth, violin & cello [WP] Øyvind Torvund: Neon Forest Space clarinet, percussion, electric guitar, cello & electronics [AP] - interval - Ekrem Mülayim: and we chart the topography of a moment solo percussion [WP] Yannis Kyriakides: Zeimbekiko 1918 violin, electric guitar & electronics [AP] Simon Steen-Andersen: On And Off And To And Fro clarinet, vibraphone, cello & 3 megaphones [AP] WP – World Premiere AP – Australian Premiere performers Lamorna Nightingale: flute Jason Noble: clarinet Claire Edwardes: percussion Zubin Kanga: piano James Crabb: conductor/accordion Zane Banks: electric guitar Veronique Serret: violin Geoffrey Gartner: cello ABOUT THE PERFORMANCE Ensemble Offspring has always Thanks to John Cage there are now taken pleasure in introducing the several decades of rich and varied unheard to Australian audiences. sound-based practices that have In New Radicals we have put to- helped dispel the idea that the gether a program of international production of music is necessarily composers whose sounds we be- the output of conventional Western lieve represent a fresh and optimis- instruments. What is interesting in tic assessment of the music of our many of the works in this program, time. All are unashamedly explora- however, is the highly notated tory in their search for new ways to reassessment of those liberated construct and receive music. And sounds back into combination with although all have now-established traditional instruments. -

New Radicals, You Get What You Give
New Radicals, You Get What You Give Wake up kids We've got the dreamers disease Age 14 we got you down on your knees So polite, you're busy still saying please Frienemies, who when you're down ain't your friend Every night we smash their Mercedes-Benz First we run; and then we laugh till we cry But when the night is falling and you cannot find the light If you feel your dream is dying Hold tight Chorus: You've got the music in you Don't let go You've got the music in you One dance left This world is gonna pull through Don't give up You've got a reason to live Can't forget you only get what you give Four a.m. we ran a miracle mile we're flat broke but hey we do it in style The bad rich God's flying in for your trial But when the night is falling and you cannot find a friend You feel your tree is breaking Just then (chorus) This whole damn world can fall apart You'll be ok, follow your heart You're in harms way I'm right behind Now say you're mine (chorus) Don't let go We feel the music in you Fly high What's real can't die You only get what you give Just don't be afraid to leave Health insurance rip off lying FDA big bankers buying Fake computer crashes dining Cloning while they're multiplying Fashion mag shoots with the aid of 8 dust brothers, Beck Hanson Courtney Love, and Marilyn Manson You're all fakes Run to your mansions Come around We'll kick your ass in! Don't let go One dance left Postscript: Championed by a soulless media misleading pople unaware they're bleeding No one with a brain is believing It's so sad you lost the meaning Never knew it anyway Human nature's so predictable I'm a fool to do your dirty work whoa, whoa New Radicals - You Get What You Give w Teksciory.pl. -

Mike's Acoustic Songlist
2 Pac California Love 3 Doors Down Here Without You Kryptonite Let Me Go Loser 30s Don't Sit Under the Apple Tree Let Me Call You Sweetheart Old Cape Cod Pistol Packin Mama Take Me Out to the Ball Game 311 All Mixed Up Amber Do You Right Flowing I'll Be Here Awhile Love Song 38 Special Caught Up in You Hold on Loosely 4 Non Blondes What's Up Adele Rolling in the Deep Set Fire to the Rain Aerosmith Living on the Edge Rag Doll Sweet Emotion What It Takes Aha Take on Me Al Green Let's Stay Together Alabama Dixieland Delight Alan Jackson It's 5 O'clock Somewhere Alan Parsons Project Eye in the Sky Alanis Morrisette You Oughta Know Alice In Chains Down Man in the Box No Excuses Nutshell Rooster Allman Bros Melissa Ramblin Man Midnight Rider America Horse with no Name Sandman Sister Golden Hair Tin Man American Authors Best Day of My Life Amy Winehouse Valerie Andy Grammer Fine By Me Honey I'm Good Keep Your Head Up Andy Williams Godfather Theme Annie Lennox Here Comes the Rain Arlo Guthrie City of New Orleans Audioslave Like a Stone Avril Lavigne I'm With You Bachman Turner Overdrive Let It Ride Backstreet Boys As Long As You Love Me I Want It That Way Bad Company Ready For Love Shooting Star Badfinger No Matter What Band The Weight Baltimora Tarzan Boy Barefoot Truth Changes in the Weather Barenaked Ladies 1,000,000 Dollars Brian Wilson Call and Answer Old Apartment What a Good Boy Barry Manilow Mandy Barry White Can't Get Enough of Your Love Bastille Pompeii Beach Boys Fun, Fun, Fun Beatles Eleanor Rigby Fool on the Hill Hard Day's Night Here Comes the Sun Hey Jude Hide Your Love Away I Saw Her Standing There I Wanna Hold Your Hand I'll Follow the Sun Julia Let it Be Norweigan Wood Obladi Oblada She Came Into the Bathroom Things We Said Today Ticket to Ride We Can Work It Out When I'm Sixty-Four With a Little Help From My Friends Bee Gees Jive Talkin Islands in the Stream Massachusetts Nights on Broadway Stayin Alive To Love Somebody Tragedy Bellamy Bros. -
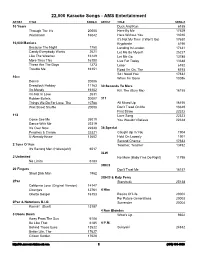
2 Column Indented
22,000 Karaoke Songs - AMS Entertainment ARTIST TITLE SONG # ARTIST TITLE SONG # 10 Years Duck And Run 6188 Through The Iris 20005 Here By Me 17629 Wasteland 16042 Here Without You 13010 It's Not My Time (I Won't Go) 17630 10,000 Maniacs Kryptonite 6190 Because The Night 1750 Landing In London 17631 Candy Everybody Wants 2621 Let Me Be Myself 25227 Like The Weather 16149 Let Me Go 13785 More Than This 16150 Live For Today 13648 These Are The Days 1273 Loser 6192 Trouble Me 16151 Road I'm On, The 6193 So I Need You 17632 10cc When I'm Gone 13086 Donna 20006 Dreadlock Holiday 11163 30 Seconds To Mars I'm Mandy 16152 Kill, The (Bury Me) 16155 I'm Not In Love 2631 Rubber Bullets 20007 311 Things We Do For Love, The 10788 All Mixed Up 16156 Wall Street Shuffle 20008 Don't Tread On Me 13649 First Straw 22322 112 Love Song 22323 Come See Me 25019 You Wouldn't Believe 22324 Dance With Me 22319 It's Over Now 22320 38 Special Peaches & Cream 22321 Caught Up In You 1904 U Already Know 13602 Hold On Loosely 1901 Second Chance 17633 2 Tons O' Fun Teacher, Teacher 13492 It's Raining Men (Hallelujah!) 6017 3LW 2 Unlimited No More (Baby I'ma Do Right) 11795 No Limits 6183 3Oh!3 20 Fingers Don't Trust Me 16157 Short Dick Man 1962 3OH!3 & Katy Perry 2Pac Starstrukk 25138 California Love (Original Version) 14147 Changes 12761 4 Him Ghetto Gospel 16153 Basics Of Life 20002 For Future Generations 20003 2Pac & Notorious B.I.G. -

Songs by Title
Songs by Title Title Artist Title Artist #1 Crush Garbage 1990 (French) Leloup (Can't Stop) Giving You Up Kylie Minogue 1994 Jason Aldean (Ghost) Riders In The Sky The Outlaws 1999 Prince (I Called Her) Tennessee Tim Dugger 1999 Prince And Revolution (I Just Want It) To Be Over Keyshia Cole 1999 Wilkinsons (If You're Not In It For Shania Twain 2 Become 1 The Spice Girls Love) I'm Outta Here 2 Faced Louise (It's Been You) Right Down Gerry Rafferty 2 Hearts Kylie Minogue The Line 2 On (Explicit) Tinashe And Schoolboy Q (Sitting On The) Dock Of Otis Redding 20 Good Reasons Thirsty Merc The Bay 20 Years And Two Lee Ann Womack (You're Love Has Lifted Rita Coolidge Husbands Ago Me) Higher 2000 Man Kiss 07 Nov Beyonce 21 Guns Green Day 1 2 3 4 Plain White T's 21 Questions 50 Cent And Nate Dogg 1 2 3 O Leary Des O' Connor 21st Century Breakdown Green Day 1 2 Step Ciara And Missy Elliott 21st Century Girl Willow Smith 1 2 Step Remix Force Md's 21st Century Girls 21st Century Girls 1 Thing Amerie 22 Lily Allen 1, 2 Step Ciara 22 Taylor Swift 1, 2, 3, 4 Feist 22 (Twenty Two) Taylor Swift 10 Days Late Third Eye Blind 22 Steps Damien Leith 10 Million People Example 23 Mike Will Made-It, Miley 10 Seconds Jazmine Sullivan Cyrus, Wiz Khalifa And 100 Years Five For Fighting Juicy J 100 Years From Now Huey Lewis And The News 24 Jem 100% Cowboy Jason Meadows 24 Hour Party People Happy Mondays 1000 Stars Natalie Bassingthwaighte 24 Hours At A Time The Marshall Tucker Band 10000 Nights Alphabeat 24 Hours From Tulsa Gene Pitney 1-2-3 Gloria Estefan 24 Hours From You Next Of Kin 1-2-3 Len Berry 2-4-6-8 Motorway Tom Robinson Band 1234 Sumptin' New Coolio 24-7 Kevon Edmonds 15 Minutes Rodney Atkins 25 Miles Edwin Starr 15 Minutes Of Shame Kristy Lee Cook 25 Minutes To Go Johnny Cash 16th Avenue Lacy J Dalton 25 Or 6 To 4 Chicago 18 And Life Skid Row 29 Nights Danni Leigh 18 Days Saving Abel 3 Britney Spears 18 Til I Die Bryan Adams 3 A.M. -

The Ultimate Playlist This List Comprises More Than 3000 Songs (Song Titles), Which Have Been Released Between 1931 and 2018, Ei
The Ultimate Playlist This list comprises more than 3000 songs (song titles), which have been released between 1931 and 2021, either as a single or as a track of an album. However, one has to keep in mind that more than 300000 songs have been listed in music charts worldwide since the beginning of the 20th century [web: http://tsort.info/music/charts.htm]. Therefore, the present selection of songs is obviously solely a small and subjective cross-section of the most successful songs in the history of modern music. Band / Musician Song Title Released A Flock of Seagulls I ran 1982 Wishing 1983 Aaliyah Are you that somebody 1998 Back and forth 1994 More than a woman 2001 One in a million 1996 Rock the boat 2001 Try again 2000 ABBA Chiquitita 1979 Dancing queen 1976 Does your mother know? 1979 Eagle 1978 Fernando 1976 Gimme! Gimme! Gimme! 1979 Honey, honey 1974 Knowing me knowing you 1977 Lay all your love on me 1980 Mamma mia 1975 Money, money, money 1976 People need love 1973 Ring ring 1973 S.O.S. 1975 Super trouper 1980 Take a chance on me 1977 Thank you for the music 1983 The winner takes it all 1980 Voulez-Vous 1979 Waterloo 1974 ABC The look of love 1980 AC/DC Baby please don’t go 1975 Back in black 1980 Down payment blues 1978 Hells bells 1980 Highway to hell 1979 It’s a long way to the top 1975 Jail break 1976 Let me put my love into you 1980 Let there be rock 1977 Live wire 1975 Love hungry man 1979 Night prowler 1979 Ride on 1976 Rock’n roll damnation 1978 Author: Thomas Jüstel -1- Rock’n roll train 2008 Rock or bust 2014 Sin city 1978 Soul stripper 1974 Squealer 1976 T.N.T. -

New Radicals You Get What You Give
You Get What You Give New Radicals One, two One, two, three Wake up kids We've got the dreamers disease Age 14 we got you down on your knees So polite, you're busy still saying please Frienemies, who when you're down ain't your friend Every night we smash their Mercedes-Benz First we run and then we laugh till we cry But when the night is falling You cannot find the light, light If you feel your dreams are dying Hold tight You've got the music in you Don't let go You've got the music in you One dance left This world is gonna pull through Don't give up You've got a reason to live Can't forget We only get what we give I'm coming home, baby You're tops Give it to me now Four a.m. we ran a miracle mile We're flat broke but hey we do it in style The bad rich God's flying in for your trial But when the night is falling You cannot find a friend, friend You feel your tree is breaking Just then You've got the music in you Don't let go You've got the music in you One dance left This world is gonna pull through Don't give up You've got a reason to live Can't forget We only get what we give This whole damn world can fall apart You'll be ok, follow your heart You're in harms way I'm right behind Now say you're mine You've got the music in you Don't let go You've got the music in you One dance left This world is gonna pull through Don't give up You've got a reason to live Can't forget We only get what we give Don't let go I feel the music in you Fly high, high What's real can't die You only get what you give You gonna get what you give Just don't be afraid to leave Health insurance rip off lying FDA big bankers buying Fake computer crashes dining Cloning while they're multiplying Fashion shoots with Beck and Hanson Courtney love and Marilyn Manson You're all fakes Run to your mansions Come around We'll kick your ass in Don't let go One dance left Don't give up Can't forget Don't Tištěno z www.texty-piesni.sk Sponzor: www.srovnavac.cz - šetříme na pojištění! Powered by TCPDF (www.tcpdf.org).