Ribonucleic Acid Export 1 Is a Kinetochore-Associated Protein That Participates in Chromosome Alignment in Mouse Oocytes
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

UNIVERSITY of CALIFORNIA, SAN DIEGO Functional Analysis of Sall4
UNIVERSITY OF CALIFORNIA, SAN DIEGO Functional analysis of Sall4 in modulating embryonic stem cell fate A dissertation submitted in partial satisfaction of the requirements for the degree Doctor of Philosophy in Molecular Pathology by Pei Jen A. Lee Committee in charge: Professor Steven Briggs, Chair Professor Geoff Rosenfeld, Co-Chair Professor Alexander Hoffmann Professor Randall Johnson Professor Mark Mercola 2009 Copyright Pei Jen A. Lee, 2009 All rights reserved. The dissertation of Pei Jen A. Lee is approved, and it is acceptable in quality and form for publication on microfilm and electronically: ______________________________________________________________ ______________________________________________________________ ______________________________________________________________ ______________________________________________________________ Co-Chair ______________________________________________________________ Chair University of California, San Diego 2009 iii Dedicated to my parents, my brother ,and my husband for their love and support iv Table of Contents Signature Page……………………………………………………………………….…iii Dedication…...…………………………………………………………………………..iv Table of Contents……………………………………………………………………….v List of Figures…………………………………………………………………………...vi List of Tables………………………………………………….………………………...ix Curriculum vitae…………………………………………………………………………x Acknowledgement………………………………………………….……….……..…...xi Abstract………………………………………………………………..…………….....xiii Chapter 1 Introduction ..…………………………………………………………………………….1 Chapter 2 Materials and Methods……………………………………………………………..…12 -

Viral Strategies to Arrest Host Mrna Nuclear Export
Viruses 2013, 5, 1824-1849; doi:10.3390/v5071824 OPEN ACCESS viruses ISSN 1999-4915 www.mdpi.com/journal/viruses Review Nuclear Imprisonment: Viral Strategies to Arrest Host mRNA Nuclear Export Sharon K. Kuss *, Miguel A. Mata, Liang Zhang and Beatriz M. A. Fontoura Department of Cell Biology, University of Texas Southwestern Medical Center, Dallas, TX 75390, USA; E-Mails: [email protected] (M.A.M.); [email protected] (L.Z.); [email protected] (B.M.A.F) * Author to whom correspondence should be addressed; E-Mail: [email protected]; Tel.: +1-214-633-2001; Fax: +1-214-648-5814. Received: 10 June 2013; in revised form: 27 June 2013 / Accepted: 11 July 2013 / Published: 18 July 2013 Abstract: Viruses possess many strategies to impair host cellular responses to infection. Nuclear export of host messenger RNAs (mRNA) that encode antiviral factors is critical for antiviral protein production and control of viral infections. Several viruses have evolved sophisticated strategies to inhibit nuclear export of host mRNAs, including targeting mRNA export factors and nucleoporins to compromise their roles in nucleo-cytoplasmic trafficking of cellular mRNA. Here, we present a review of research focused on suppression of host mRNA nuclear export by viruses, including influenza A virus and vesicular stomatitis virus, and the impact of this viral suppression on host antiviral responses. Keywords: virus; influenza virus; vesicular stomatitis virus; VSV; NS1; matrix protein; nuclear export; nucleo-cytoplasmic trafficking; mRNA export; NXF1; TAP; CRM1; Rae1 1. Introduction Nucleo-cytoplasmic trafficking of proteins and RNA is critical for proper cellular functions and survival. -

F-Box Protein RAE1 Regulates the Stability of the Aluminum-Resistance Transcription Factor STOP1 in Arabidopsis
F-box protein RAE1 regulates the stability of the aluminum-resistance transcription factor STOP1 in Arabidopsis Yang Zhanga,b,1, Jie Zhanga,1, Jinliang Guoa,b,1, Fanglin Zhoua, Somesh Singha, Xuan Xub, Qi Xiec, Zhongbao Yangd, and Chao-Feng Huanga,b,2 aNational Key Laboratory of Plant Molecular Genetics, Shanghai Center for Plant Stress Biology, Center of Excellence for Molecular Plant Sciences, Chinese Academy of Sciences, 200032 Shanghai, China; bCollege of Resources and Environmental Sciences, Nanjing Agricultural University, 210095 Nanjing, China; cState Key Laboratory of Plant Genomics, Institute of Genetics and Developmental Biology, Chinese Academy of Sciences, 100101 Beijing, China; and dKey Laboratory of Plant Cell Engineering and Germplasm Innovation, Ministry of Education, College of Life Science, Shandong University, 250100 Jinan, China Edited by Luis Herrera-Estrella, Center for Research and Advanced Studies, Irapuato, Mexico, and approved November 19, 2018 (received for review August 21, 2018) Aluminum (Al) toxicity is a major factor limiting crop production expression of STOP1-downstream Al-resistance genes, including on acid soils, which represent over 30% of the world’s arable land. AtALMT1, AtMATE, and ALS3, is induced by Al (8, 12). These Some plants have evolved mechanisms to detoxify Al. Arabidopsis, results suggest the possibility that STOP1 might be regulated by for example, secretes malate via the AtALMT1 transporter to che- Al at posttranscriptional or posttranslational levels. late and detoxify Al. The C2H2-type transcription factor STOP1 plays a Ubiquitin-mediated protein degradation is an important post- crucial role in Al resistance by inducing the expression of a set of translational mechanism that regulates numerous biological processes genes, including AtALMT1. -

BUB3 That Dissociates from BUB1 Activates Caspase-Independent Mitotic Death (CIMD)
Cell Death and Differentiation (2010) 17, 1011–1024 & 2010 Macmillan Publishers Limited All rights reserved 1350-9047/10 $32.00 www.nature.com/cdd BUB3 that dissociates from BUB1 activates caspase-independent mitotic death (CIMD) Y Niikura1, H Ogi1, K Kikuchi1 and K Kitagawa*,1 The cell death mechanism that prevents aneuploidy caused by a failure of the spindle checkpoint has recently emerged as an important regulatory paradigm. We previously identified a new type of mitotic cell death, termed caspase-independent mitotic death (CIMD), which is induced during early mitosis by partial BUB1 (a spindle checkpoint protein) depletion and defects in kinetochore–microtubule attachment. In this study, we have shown that survived cells that escape CIMD have abnormal nuclei, and we have determined the molecular mechanism by which BUB1 depletion activates CIMD. The BUB3 protein (a BUB1 interactor and a spindle checkpoint protein) interacts with p73 (a homolog of p53), specifically in cells wherein CIMD occurs. The BUB3 protein that is freed from BUB1 associates with p73 on which Y99 is phosphorylated by c-Abl tyrosine kinase, resulting in the activation of CIMD. These results strongly support the hypothesis that CIMD is the cell death mechanism protecting cells from aneuploidy by inducing the death of cells prone to substantial chromosome missegregation. Cell Death and Differentiation (2010) 17, 1011–1024; doi:10.1038/cdd.2009.207; published online 8 January 2010 Aneuploidy – the presence of an abnormal number of of spindle checkpoint activity.20,21 -

Patronus Is the Elusive Plant Securin, Preventing Chromosome Separation By
bioRxiv preprint doi: https://doi.org/10.1101/606285; this version posted April 11, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. 1 Patronus is the elusive plant securin, preventing chromosome separation by 2 antagonizing separase 3 Laurence Cromer1, Sylvie Jolivet1, Dipesh Kumar Singh1, Floriane Berthier1, Nancy 4 De Winne2, Geert De Jaeger2, Shinichiro Komaki3,4, Maria Ada Prusicki3, Arp 5 Schnittger3, Raphael Guérois5 and Raphael Mercier1* 6 7 1 Institut Jean-Pierre Bourgin, UMR1318 INRA-AgroParisTech, Université Paris- 8 Saclay, RD10, 78000 Versailles, France. 9 2 Department of Plant Biotechnology and Bioinformatics, Ghent University, Ghent, 10 Belgium, VIB Center for Plant Systems Biology, Ghent, Belgium 11 3 Department of Developmental Biology, Institute for Plant Sciences and 12 Microbiology, University of Hamburg, 22609 Hamburg, Germany. 13 4 Present address : Nara Institute of Science and Technology, Graduate School of 14 Biological Sciences, 8916-5 Takayama, Ikoma, Nara 630-0192, Japan 15 5 Institute for Integrative Biology of the Cell (I2BC), Commissariat à l’Energie 16 Atomique et aux Energies Alternatives (CEA), Centre National de la Recherche 17 Scientifique (CNRS), Université Paris-Sud, CEA-Saclay, Gif-sur-Yvette, France 18 * Corresponding author. [email protected] 19 1 bioRxiv preprint doi: https://doi.org/10.1101/606285; this version posted April 11, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. -
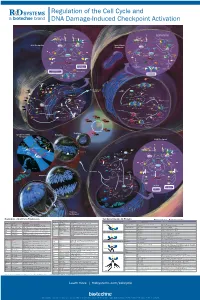
Regulation of the Cell Cycle and DNA Damage-Induced Checkpoint Activation
RnDSy-lu-2945 Regulation of the Cell Cycle and DNA Damage-Induced Checkpoint Activation IR UV IR Stalled Replication Forks/ BRCA1 Rad50 Long Stretches of ss-DNA Rad50 Mre11 BRCA1 Nbs1 Rad9-Rad1-Hus1 Mre11 RPA MDC1 γ-H2AX DNA Pol α/Primase RFC2-5 MDC1 Nbs1 53BP1 MCM2-7 53BP1 γ-H2AX Rad17 Claspin MCM10 Rad9-Rad1-Hus1 TopBP1 CDC45 G1/S Checkpoint Intra-S-Phase RFC2-5 ATM ATR TopBP1 Rad17 ATRIP ATM Checkpoint Claspin Chk2 Chk1 Chk2 Chk1 ATR Rad50 ATRIP Mre11 FANCD2 Ubiquitin MDM2 MDM2 Nbs1 CDC25A Rad50 Mre11 BRCA1 Ub-mediated Phosphatase p53 CDC25A Ubiquitin p53 FANCD2 Phosphatase Degradation Nbs1 p53 p53 CDK2 p21 p21 BRCA1 Ub-mediated SMC1 Degradation Cyclin E/A SMC1 CDK2 Slow S Phase CDC45 Progression p21 DNA Pol α/Primase Slow S Phase p21 Cyclin E Progression Maintenance of Inhibition of New CDC6 CDT1 CDC45 G1/S Arrest Origin Firing ORC MCM2-7 MCM2-7 Recovery of Stalled Replication Forks Inhibition of MCM10 MCM10 Replication Origin Firing DNA Pol α/Primase ORI CDC6 CDT1 MCM2-7 ORC S Phase Delay MCM2-7 MCM10 MCM10 ORI Geminin EGF EGF R GAB-1 CDC6 CDT1 ORC MCM2-7 PI 3-Kinase p70 S6K MCM2-7 S6 Protein Translation Pre-RC (G1) GAB-2 MCM10 GSK-3 TSC1/2 MCM10 ORI PIP2 TOR Promotes Replication CAK EGF Origin Firing Origin PIP3 Activation CDK2 EGF R Akt CDC25A PDK-1 Phosphatase Cyclin E/A SHIP CIP/KIP (p21, p27, p57) (Active) PLCγ PP2A (Active) PTEN CDC45 PIP2 CAK Unwinding RPA CDC7 CDK2 IP3 DAG (Active) Positive DBF4 α Feedback CDC25A DNA Pol /Primase Cyclin E Loop Phosphatase PKC ORC RAS CDK4/6 CDK2 (Active) Cyclin E MCM10 CDC45 RPA IP Receptor -

Control of the Cell Cycle During Meiotic Maturation of Mouse Oocytes
Control of the cell cycle during meiotic maturation of mouse oocytes Ibtissem Nabti Department of Cell and Developmental Biology University College London London, UK A thesis submitted for the degree of Doctor of Philosophy April 2010 Declaration The work presented in this thesis was conducted under the supervision of Professor John Carroll in the department of cell and developmental biology, University College London, and Professor Keith Jones in the department of reproductive physiology, University of Newcastle upon Tyne. I, Ibtissem Nabti confirm that the data presented in this thesis is my own and from original studies. Also, this dissertation has not previously been submitted, wholly or in part, to any other university for any degree or diploma. Ibtissem Nabti 1 Abstract The overall aim of the work presented in this thesis is to better understand the molecular mechanisms underlying the progression of the meiotic division in mammalian oocytes. The first goal of this project was to investigate the relative contribution of securin and cyclin B1 to the control of the protease separase during the indeterminate period of metaphase II arrest. I found here that although there are conditions in which either securin or MPF can prevent chromosome disjunction, such as during meiosis I, securin is the predominant inhibitor of separase during metaphase II arrest. Thus, CDK1 pharmacological inhibition as well as antibody inhibition of CDK1/cyclin B1 binding to separase, both failed to induce sister chromatid disjunction. Instead, securin morpholino knockdown induced sister chromatid separation, which could be rescued by injection of securin cRNA. I also examined the effect of CDK1 and MAPK activities on securin stability. -

Snapshot: the Nuclear Envelope II Andrea Rothballer and Ulrike Kutay Institute of Biochemistry, ETH Zurich, 8093 Zurich, Switzerland
SnapShot: The Nuclear Envelope II Andrea Rothballer and Ulrike Kutay Institute of Biochemistry, ETH Zurich, 8093 Zurich, Switzerland H. sapiens D. melanogaster C. elegans S. pombe S. cerevisiae Cytoplasmic filaments RanBP2 (Nup358) Nup358 CG11856 NPP-9 – – – Nup214 (CAN) DNup214 CG3820 NPP-14 Nup146 SPAC23D3.06c Nup159 Cytoplasmic ring and Nup88 Nup88 (Mbo) CG6819 – Nup82 SPBC13A2.02 Nup82 associated factors GLE1 GLE1 CG14749 – Gle1 SPBC31E1.05 Gle1 hCG1 (NUP2L1, NLP-1) tbd CG18789 – Amo1 SPBC15D4.10c Nup42 (Rip1) Nup98 Nup98 CG10198 Npp-10N Nup189N SPAC1486.05 Nup145N, Nup100, Nup116 Nup 98 complex RAE1 (GLE2) Rae1 CG9862 NPP-17 Rae1 SPBC16A3.05 Gle2 (Nup40) Nup160 Nup160 CG4738 NPP-6 Nup120 SPBC3B9.16c Nup120 Nup133 Nup133 CG6958 NPP-15 Nup132, Nup131 SPAC1805.04, Nup133 SPBP35G2.06c Nup107 Nup107 CG6743 NPP-5 Nup107 SPBC428.01c Nup84 Nup96 Nup96 CG10198 NPP-10C Nup189C SPAC1486.05 Nup145C Outer NPC scaffold Nup85 (PCNT1) Nup75 CG5733 NPP-2 Nup-85 SPBC17G9.04c Nup85 (Nup107-160 complex) Seh1 Nup44A CG8722 NPP-18 Seh1 SPAC15F9.02 Seh1 Sec13 Sec13 CG6773 Npp-20 Sec13 SPBC215.15 Sec13 Nup37 tbd CG11875 – tbd SPAC4F10.18 – Nup43 Nup43 CG7671 C09G9.2 – – – Centrin-21 tbd CG174931, CG318021 R08D7.51 Cdc311 SPCC1682.04 Cdc311 Nup205 tbd CG11943 NPP-3 Nup186 SPCC290.03c Nup192 Nup188 tbd CG8771 – Nup184 SPAP27G11.10c Nup188 Central NPC scaffold Nup155 Nup154 CG4579 NPP-8 tbd SPAC890.06 Nup170, Nup157 (Nup53-93 complex) Nup93 tbd CG7262 NPP-13 Nup97, Npp106 SPCC1620.11, Nic96 SPCC1739.14 Nup53(Nup35, MP44) tbd CG6540 NPP-19 Nup40 SPAC19E9.01c -

RAE1 Ligands for the NKG2D Receptor Are Regulated by STING-Dependent DNA Sensor Pathways in Lymphoma
Published OnlineFirst March 3, 2014; DOI: 10.1158/0008-5472.CAN-13-1703 Cancer Microenvironment and Immunology Research RAE1 Ligands for the NKG2D Receptor Are Regulated by STING-Dependent DNA Sensor Pathways in Lymphoma Adeline R. Lam1,2,NinaLeBert1,SamanthaS.W.Ho1,YuJ.Shen1,2, Melissa L.F. Tang1,GordonM.Xiong1, J. Ludovic Croxford1, Christine X. Koo1,2,3,4,KenJ.Ishii3,4,ShizuoAkira5,DavidH.Raulet6, and Stephan Gasser1,2 Abstract The immunoreceptor NKG2D originally identified in natural killer (NK) cells recognizes ligands that are upregulated on tumor cells. Expression of NKG2D ligands (NKG2DL) is induced by the DNA damage response (DDR), which is often activated constitutively in cancer cells, revealing them to NK cells as a mechanism of immunosurveillance. Here, we report that the induction of retinoic acid early transcript 1 (RAE1) ligands for NKG2D by the DDR relies on a STING-dependent DNA sensor pathway involving the effector molecules TBK1 and IRF3. Cytosolic DNA was detected in lymphoma cell lines that express RAE1 and its occurrence required activation of the DDR. Transfection of DNA into ligand-negative cells was sufficient to induce RAE1 expression. þ À Irf3 / ;Em-Myc mice expressed lower levels of RAE1 on tumor cells and showed a reduced survival rate compared þ þ with Irf3 / ;Em-Myc mice. Taken together, our results suggest that genomic damage in tumor cells leads to activation of STING-dependent DNA sensor pathways, thereby activating RAE1 and enabling tumor immuno- surveillance. Cancer Res; 74(8); 1–11. Ó2014 AACR. Introduction DNA damage and the constitutive expression of NKG2DLs in The NKG2D system is an arm of innate immune recognition, some tumor cell lines (6). -

The DNA Sequence and Comparative Analysis of Human Chromosome 20
articles The DNA sequence and comparative analysis of human chromosome 20 P. Deloukas, L. H. Matthews, J. Ashurst, J. Burton, J. G. R. Gilbert, M. Jones, G. Stavrides, J. P. Almeida, A. K. Babbage, C. L. Bagguley, J. Bailey, K. F. Barlow, K. N. Bates, L. M. Beard, D. M. Beare, O. P. Beasley, C. P. Bird, S. E. Blakey, A. M. Bridgeman, A. J. Brown, D. Buck, W. Burrill, A. P. Butler, C. Carder, N. P. Carter, J. C. Chapman, M. Clamp, G. Clark, L. N. Clark, S. Y. Clark, C. M. Clee, S. Clegg, V. E. Cobley, R. E. Collier, R. Connor, N. R. Corby, A. Coulson, G. J. Coville, R. Deadman, P. Dhami, M. Dunn, A. G. Ellington, J. A. Frankland, A. Fraser, L. French, P. Garner, D. V. Grafham, C. Grif®ths, M. N. D. Grif®ths, R. Gwilliam, R. E. Hall, S. Hammond, J. L. Harley, P. D. Heath, S. Ho, J. L. Holden, P. J. Howden, E. Huckle, A. R. Hunt, S. E. Hunt, K. Jekosch, C. M. Johnson, D. Johnson, M. P. Kay, A. M. Kimberley, A. King, A. Knights, G. K. Laird, S. Lawlor, M. H. Lehvaslaiho, M. Leversha, C. Lloyd, D. M. Lloyd, J. D. Lovell, V. L. Marsh, S. L. Martin, L. J. McConnachie, K. McLay, A. A. McMurray, S. Milne, D. Mistry, M. J. F. Moore, J. C. Mullikin, T. Nickerson, K. Oliver, A. Parker, R. Patel, T. A. V. Pearce, A. I. Peck, B. J. C. T. Phillimore, S. R. Prathalingam, R. W. Plumb, H. Ramsay, C. M. -

APC/C-Cdh1-Dependent Anaphase and Telophase Progression During
Toda et al. Cell Division 2012, 7:4 http://www.celldiv.com/content/7/1/4 RESEARCH Open Access APC/C-Cdh1-dependent anaphase and telophase progression during mitotic slippage Kazuhiro Toda1, Kayoko Naito1, Satoru Mase1, Masaru Ueno1,2, Masahiro Uritani1, Ayumu Yamamoto1 and Takashi Ushimaru1* Abstract Background: The spindle assembly checkpoint (SAC) inhibits anaphase progression in the presence of insufficient kinetochore-microtubule attachments, but cells can eventually override mitotic arrest by a process known as mitotic slippage or adaptation. This is a problem for cancer chemotherapy using microtubule poisons. Results: Here we describe mitotic slippage in yeast bub2Δ mutant cells that are defective in the repression of precocious telophase onset (mitotic exit). Precocious activation of anaphase promoting complex/cyclosome (APC/ C)-Cdh1 caused mitotic slippage in the presence of nocodazole, while the SAC was still active. APC/C-Cdh1, but not APC/C-Cdc20, triggered anaphase progression (securin degradation, separase-mediated cohesin cleavage, sister- chromatid separation and chromosome missegregation), in addition to telophase onset (mitotic exit), during mitotic slippage. This demonstrates that an inhibitory system not only of APC/C-Cdc20 but also of APC/C-Cdh1 is critical for accurate chromosome segregation in the presence of insufficient kinetochore-microtubule attachments. Conclusions: The sequential activation of APC/C-Cdc20 to APC/C-Cdh1 during mitosis is central to accurate mitosis. Precocious activation of APC/C-Cdh1 in metaphase (pre-anaphase) causes mitotic slippage in SAC-activated cells. For the prevention of mitotic slippage, concomitant inhibition of APC/C-Cdh1 may be effective for tumor therapy with mitotic spindle poisons in humans. -

Functional Interaction Between Bubr1 and Securin in an Anaphase- Promoting Complex/Cyclosomecdc20–Independent Manner
Research Article Functional Interaction between BubR1 and Securin in an Anaphase- Promoting Complex/CyclosomeCdc20–Independent Manner Hyun-Soo Kim,1 Yoon-Kyung Jeon,2 Geun-Hyoung Ha,1 Hye-Young Park,1 Yu-Jin Kim,1 Hyun-Jin Shin,1 Chang Geun Lee,1 Doo-Hyun Chung,2 and Chang-Woo Lee1 1Department of Molecular Cell Biology, Center for Molecular Medicine, Samsung Biomedical Research Institute, Sungkyunkwan University School of Medicine, Suwon, Gyeonggi, Korea and 2Department of Pathology, Seoul National University College of Medicine, Seoul, Korea Abstract Cdc20 (APC/CCdc20). However, Cdc20 in MCC is unable to acti- vate APC/C, as shown by several experiments in which the addi- Activation of the mitotic checkpoint requires the precise tion of Mad2 and/or BubR1 leads to the checkpoint-mediated timing and spatial organization of mitotic regulatory inhibition of APC/CCdc20 activity (2, 10, 11). Silencing of this events, and ensures accurate chromosome segregation. checkpoint signal is initiated by the release of the inhibitory Mitotic checkpoint proteins such as BubR1 and Mad2 bind mitotic checkpoint protein complex from APC/CCdc20; APC/CCdc20 to Cdc20, and inhibit anaphase-promoting complex/cyclo- can drive cells into anaphase by inducing the degradation of someCdc20–mediated securin degradation and the onset of securin (pituitary tumor-transforming gene, PTTG), a small protein anaphase. BubR1 mediates the proper attachment of micro- that inhibits the protease separase and mitotic cyclins (12–15). tubules to kinetochores, and links the regulation of chromo- In metaphase-to-anaphase transition, APC/CCdc20 initiates the some-spindle attachment to mitotic checkpoint signaling. process of chromosome segregation through ubiquitination of Therefore, disruption of BubR1 activity results in a loss securin.