DNA & RNA Precipitation Solutions
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Stock Solutions
STOCK SOLUTIONS 5 M NaCl (F.W. 58.44) 500 ml: 146.1 g NaCl ~350 ml H2O Dissolve, then bring up to volume with H2O Sterilize by autoclaving (15 minutes) 1 M Tris, pH 8 (F.W. 121.1) 500 ml: 60.55 g Trizma base ~400 ml H2O Approximately 21.1 ml concentrated HCl (Use pH meter) Bring up to volume with H2O Sterilize by autoclaving (15 minutes) 1 M Tris, pH 7.6 (F.W. 121.1) 500 ml: 60.55 g Trizma base ~400 ml H2O Approximately 28.5 ml concentrated HCl (Use pH meter) Bring up to volume with H2O Sterilize by autoclaving (15 minutes) 1 M MgCl2 (F.W. 203.30) 100 ml: 20.33 g MgCl2 ~70 ml H2O Dissolve, then bring up to volume with H2O Sterilize by autoclaving (15 minutes) 1 M CaCl2 (F.W. 147.02) 100 ml: 14.70 g CaCl2 ~90 ml H2O Dissolve, then bring up to volume with H2O Sterilization not required 1 M MgSO4 (F.W. 246.47) 100 ml: 24.65 g MgSO4 ~75 ml H2O Dissolve, then bring up to volume with H2O Sterilize by autoclaving (15 minutes) 0.1 M MgCl2 (F.W. 203.30) 100 ml: 2.03 g MgCl2 ~95 ml H2O Dissolve, then bring up to volume with H2O Sterilize by autoclaving (15 minutes) 3 M NaOH (F.W. 40.00) 100 ml: 12.0 g NaOH ~80 ml H2O Dissolve, then bring up to volume with H2O Sterilization not required 2 M Sorbitol (F.W. 182.2) 500 ml: 182.2 g Sorbitol ~300 ml H2O Dissolve, then bring up to volume with H2O Sterilization by filtration 5 M Potassium acetate (F.W. -

NON-HAZARDOUS CHEMICALS May Be Disposed of Via Sanitary Sewer Or Solid Waste
NON-HAZARDOUS CHEMICALS May Be Disposed Of Via Sanitary Sewer or Solid Waste (+)-A-TOCOPHEROL ACID SUCCINATE (+,-)-VERAPAMIL, HYDROCHLORIDE 1-AMINOANTHRAQUINONE 1-AMINO-1-CYCLOHEXANECARBOXYLIC ACID 1-BROMOOCTADECANE 1-CARBOXYNAPHTHALENE 1-DECENE 1-HYDROXYANTHRAQUINONE 1-METHYL-4-PHENYL-1,2,5,6-TETRAHYDROPYRIDINE HYDROCHLORIDE 1-NONENE 1-TETRADECENE 1-THIO-B-D-GLUCOSE 1-TRIDECENE 1-UNDECENE 2-ACETAMIDO-1-AZIDO-1,2-DIDEOXY-B-D-GLYCOPYRANOSE 2-ACETAMIDOACRYLIC ACID 2-AMINO-4-CHLOROBENZOTHIAZOLE 2-AMINO-2-(HYDROXY METHYL)-1,3-PROPONEDIOL 2-AMINOBENZOTHIAZOLE 2-AMINOIMIDAZOLE 2-AMINO-5-METHYLBENZENESULFONIC ACID 2-AMINOPURINE 2-ANILINOETHANOL 2-BUTENE-1,4-DIOL 2-CHLOROBENZYLALCOHOL 2-DEOXYCYTIDINE 5-MONOPHOSPHATE 2-DEOXY-D-GLUCOSE 2-DEOXY-D-RIBOSE 2'-DEOXYURIDINE 2'-DEOXYURIDINE 5'-MONOPHOSPHATE 2-HYDROETHYL ACETATE 2-HYDROXY-4-(METHYLTHIO)BUTYRIC ACID 2-METHYLFLUORENE 2-METHYL-2-THIOPSEUDOUREA SULFATE 2-MORPHOLINOETHANESULFONIC ACID 2-NAPHTHOIC ACID 2-OXYGLUTARIC ACID 2-PHENYLPROPIONIC ACID 2-PYRIDINEALDOXIME METHIODIDE 2-STEP CHEMISTRY STEP 1 PART D 2-STEP CHEMISTRY STEP 2 PART A 2-THIOLHISTIDINE 2-THIOPHENECARBOXYLIC ACID 2-THIOPHENECARBOXYLIC HYDRAZIDE 3-ACETYLINDOLE 3-AMINO-1,2,4-TRIAZINE 3-AMINO-L-TYROSINE DIHYDROCHLORIDE MONOHYDRATE 3-CARBETHOXY-2-PIPERIDONE 3-CHLOROCYCLOBUTANONE SOLUTION 3-CHLORO-2-NITROBENZOIC ACID 3-(DIETHYLAMINO)-7-[[P-(DIMETHYLAMINO)PHENYL]AZO]-5-PHENAZINIUM CHLORIDE 3-HYDROXYTROSINE 1 9/26/2005 NON-HAZARDOUS CHEMICALS May Be Disposed Of Via Sanitary Sewer or Solid Waste 3-HYDROXYTYRAMINE HYDROCHLORIDE 3-METHYL-1-PHENYL-2-PYRAZOLIN-5-ONE -

Methanogenesis Rates in Acetate and Nitrate Amended Anoxic Slurries
Methanogenesis rates in acetate and nitrate amended anoxic slurries BIOS 35502: Practicum in Environmental Field Biology Patrick Revord Advisor: William West 2011 Abstract With increasing urbanization and land use changes, pollution of lakes and wetland ecosystems is imminent. Any influx of nutrients, anthropogenic or natural, can have dramatic effects on lake gas production and flux. However, the net effect of simultaneous increase of both acetate and nitrate is unknown. Methane (CH4) production was measured in anoxic sediment and water slurries amended with ammonium nitrate (NH4NO3), which has been shown to inhibit methanogenesis, and sodium acetate (CH3COONa or NaOAc), which is known to increase methanogenesis. The addition of acetate significantly increased the methanogenesis rate, but the nitrate amendment had no significant effect. The simultaneous amendment of both acetate and nitrate showed no significant increase in CH4 compared to the control, indicating that the presence of nitrate may have reduced the effect of acetate amendment. Introduction Methane, a greenhouse gas associated with global warming, continues to increase in concentration in our atmosphere. Global yearly flux of methane into the atmosphere is 566 teragrams of CH4 per year, which is more than double pre-industrial yearly flux (Solomon et al. 2007). Increasing urbanization and land-use changes contribute significantly to increased gas levels (Anderson et al. 2010, Vitousek 1994). Nutrients travel from anthropogenic sources such as wastewater treatment facilities, landfills, and agricultural plots into nearby lakes, rivers, and wetlands, causing increased primary productivity in a process known as eutrophication (Vitousek et al. 1997). The increased nutrients and productivity lead to toxic algal blooms that create products such as acetate, H2, and CO2; a nutrient-rich anoxic environment suitable for anaerobic bacteria to produce unnaturally high levels of methane and other greenhouse gases (Davis and Koop 2006, West unpublished data). -

Potassium Acetate
OKI GROUNDWATER COMMITTEE June 3, 2009 - 10:00 A.M. OKI Board Room 720 East Pete Rose Way (at the corner of Eggleston Avenue)* AGENDA 1. Welcome/Introductions 2. Announcements 3. OKI Staff Updates 4. Update on Local Groundwater Management Efforts Frank Bell, Mike Heuer, Greg Stanley, Bruce Whitteberry, Tom Yeager 5. The St. Clair Township Comprehensive Plan: Protecting Source Water and Community Character Jane Wittke, OKI 6. The Groundwater Implications of Geothermal Systems: Geothermal Systems in Ohio Russell Smith, Ohio Department of Health and Geothermal System Construction Jacob Crabtree, Crabtree Drilling Company 7. Other Business ADJOURNMENT ** SEE the MAP and DIRECTIONS on the REVERSE HARD COPY (or in separate attachment to email) Mill Creek Headwaters Project Funding Federal Funds: $498,000 from Ohio EPA under Section 319 of the Clean Water Act Local Match: $472,600 from West Chester Township’s conservation easements and project partners’ in-kind services Mill Creek Headwaters Project Purposes 1. Educate public on how to reduce water pollution 2. Evaluate best management practices 3. Reduce nonpoint source pollution by installing: • 400 feet of stabilized streambank • 3,000 feet of restored riparian corridor • 18 acres of restored floodplain wetland Project Partners •West Chester Township • OKI Regional Council of Governments • Butler Soil and Water Conservation District • Mill Creek Watershed Council of Communities • Butler County Engineer’s Office • Butler County Water and Sewer Department • Greenacres Foundation • University -

NMR Chemical Shifts of Common Laboratory Solvents As Trace Impurities
7512 J. Org. Chem. 1997, 62, 7512-7515 NMR Chemical Shifts of Common Laboratory Solvents as Trace Impurities Hugo E. Gottlieb,* Vadim Kotlyar, and Abraham Nudelman* Department of Chemistry, Bar-Ilan University, Ramat-Gan 52900, Israel Received June 27, 1997 In the course of the routine use of NMR as an aid for organic chemistry, a day-to-day problem is the identifica- tion of signals deriving from common contaminants (water, solvents, stabilizers, oils) in less-than-analyti- cally-pure samples. This data may be available in the literature, but the time involved in searching for it may be considerable. Another issue is the concentration dependence of chemical shifts (especially 1H); results obtained two or three decades ago usually refer to much Figure 1. Chemical shift of HDO as a function of tempera- more concentrated samples, and run at lower magnetic ture. fields, than today’s practice. 1 13 We therefore decided to collect H and C chemical dependent (vide infra). Also, any potential hydrogen- shifts of what are, in our experience, the most popular bond acceptor will tend to shift the water signal down- “extra peaks” in a variety of commonly used NMR field; this is particularly true for nonpolar solvents. In solvents, in the hope that this will be of assistance to contrast, in e.g. DMSO the water is already strongly the practicing chemist. hydrogen-bonded to the solvent, and solutes have only a negligible effect on its chemical shift. This is also true Experimental Section for D2O; the chemical shift of the residual HDO is very NMR spectra were taken in a Bruker DPX-300 instrument temperature-dependent (vide infra) but, maybe counter- (300.1 and 75.5 MHz for 1H and 13C, respectively). -

UNITED STATES PATENT Office
Patented Mar. 7, 1950 2499,924 UNITED STATES PATENT office 2,499,924 HIGH VIscosiTY PoLYVINYL Alcohol Edward Lavin, Springfield, Mass, assigner to Shawinigan Resins Corporation, Springfield, Mass, a corporation of Massachusetts No Drawing. Appliention November 20, 1946, Serial No. 711,207 4 Claims. (CL 260-91s), 2 This invention relates to a process for pre The process employed in preparing the prod paring polyvinyl alcohols having high viscosities. lucts set forth in the examples in Tables A and B Hydrolyzed polyvinyl acetates are useful for comprises dissolving the polyvinyl acetate in the many purposes. However, for some uses e. g., toluene and then adding the methanol containing as thickening agents, protective colloids and the the sulfuric acid (98% HaSO4) dissolved therein. like, it is desirable that such materials possess The charge is prepared in a suitable apparatus materially higher viscosities. provided with an agitator and a water-cooled It is an object of this invention to provide a return condenser. The hydrolysis is carried out process for preparing partially hydrolyzed poly by heating the charge at its reflux temperature vinyl acetates having an unusually high viscosity. O and continuing the heating under these condi A particular object of this invention is to pro tions until the desired degree of hydrolysis is vide a process for preparing high viscosity poly effected. The reaction mixture is then cooled vinyl alcohols having an acetate content of 15-40 and the hydrolyzed product which is in the form per cent by weight, calculated as polyvinyl of...a precipitate is allowed to settle. Thereafter acetate. -
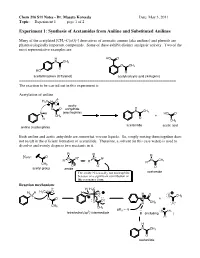
Experiment 1: Synthesis of Acetamides from Aniline and Substituted Anilines
Chem 216 S11 Notes - Dr. Masato Koreeda Date: May 3, 2011 Topic: __Experiment 1____ page 1 of 2. Experiment 1: Synthesis of Acetamides from Aniline and Substituted Anilines Many of the acetylated [CH3–C(=O)-] derivatives of aromatic amines (aka anilines) and phenols are pharmacologically important compounds. Some of these exhibit distinct analgesic activity. Two of the most representative examples are: H HO O N CH3 O CH3 O HO O acetaminophen (Tylenol) acetylsalicylic acid (Aspirin) ======================================================================= The reaction to be carried out in this experiment is: Acetylation of aniline δ- H3C δ+ O acetic O O anhydride H N CH3 N H (electrophile) HO O CH3 + H O CH3 acetanilide acetic acid aniline (nucleophile) Both aniline and acetic anhydride are somewhat viscous liquids. So, simply mixing them together does not result in the efficient formation of acetanilide. Therefore, a solvent (in this case water) is used to dissolve and evenly disperse two reactants in it. R R R Note: O + N R" N R" N CH R' R' R' 3 CH3 - O O O acetyl group amide The amide N is usually not nucleophilic acetamide because of a significant contribution of this resonance form. Reaction mechanism: δ- H H3C H H H3C δ+ O H N O H O N O CH3 O O N + O O H CH3 O CH3 CH3 pKa ~ -5 3 O CH3 tetrahedral (sp ) intermediate B (including ) O H N CH3 O acetanilide Chem 216 S11 Notes - Dr. Masato Koreeda Date: May 3, 2011 Topic: __Experiment 1____ page 2 of 2. Additional comments on the reaction mechanism: 1. -

FLUID COMPATIBILITY CHART for Metal Threaded Fittings Sealed with Loctite¨ Sealants LIQUIDS, SOLUTIONS & SUSPENSIONS
FLUID COMPATIBILITY CHART for metal threaded fittings sealed with Loctite® Sealants LIQUIDS, SOLUTIONS & SUSPENSIONS LEGEND: Bagasse Fibers.......................... Chlorobenzene Dry ................... Ferrous Chloride ...................... Ion Exclusion Glycol ................. Nickel Chloride.......................... All Loctite® Anaerobic Sealants are Barium Acetate ........................ Chloroform Dry......................... Ferrous Oxalate......................... Irish Moss Slurry...................... Nickel Cyanide ......................... Compatible Including #242®, 243, Barium Carbonate..................... Chloroformate Methyl............... Ferrous Sulfate10%.................. Iron Ore Taconite ..................... Nickel Fluoborate ..................... 542, 545, 565, 567, 569, 571, 572, Barium Chloride........................ Chlorosulfonic Acid .................. Ferrous Sulfate (Sat)................. Iron Oxide ................................ Nickel Ore Fines ....................... 577, 580, 592 Barium Hydroxide..................... Chrome Acid Cleaning .............. Fertilizer Sol ............................. Isobutyl Alcohol ....................... Nickel Plating Bright ................. † Use Loctite® #270, 271™, 277, 554 Barium Sulfate.......................... Chrome Liquor.......................... Flotation Concentrates.............. Isobutyraldehyde ..................... Nickel Sulfate ........................... Not Recommended Battery Acid .............................. Chrome Plating -

Production of Low-Cost Acetate Deicers from Biomass and Industrial Wastes
Production of Low-Cost Acetate Deicers from Biomass and Industrial Wastes Shang-Tian Yang and Zuwei Jin, The Ohio State University Brian H. ChoUar, Federal Highway Administration Calcium magnesium acetate (CMA), a mixture of calcium 1 rom 10 million to 14 million tons of road salt are acetate and magnesium acetate, is used as an environmen• I used annually in the United States and Canada. tally benign roadway deicer. The present commercial F Salt is an extremely effective snow and ice con• CMA deicer made from glacial acetic acid and dolomitic trol agent and is relatively inexpensive. However, a lime or limestone is more expensive than salt and other study in New York State showed that although 1 ton of deicers. Also, a liquid potassium acetate deicer is used to road salt cost only $25, it caused more than $1,400 in replace urea and glycol in airport runway deicing. Two al• damage (1). Salt is corrosive to concrete and metals ternative low-cost methods to produce these acetate de- used in the nation's infrastructure, is harmful to road• icers from cheap feedstocks, such as biomass and side vegetation, and poses serious threats to environ• industrial wastes, were studied. CMA deicers produced ment and ground-water quality in some regions (2). from cheese whey by fermentation and extraction were FHWA spends about $12.5 billion annually, a sub• tested for their acetate content and deicing property. The stantial portion of which is used to rebuild and resur• CMA solid sample obtained from extraction of the acetic face highways and bridges damaged by salt corrosion. -

Sodium Acetate
SODIUM ACETATE Prepared at the 18th JECFA (1974), published in NMRS 54B (1975) and in FNP 52 (1992). Metals and arsenic specifications revised at the 59th JECFA (2002). An ADI not limited' was established at the 17th JECFA (1973) SYNONYMS INS No. 262(i) DEFINITION Chemical names Sodium acetate C.A.S. number 127-09-3 Chemical formula C2H3NaO2 · nH2O (n = 0 or 3) Structural formula CH3COONa · nH2O (n = 0 or 3) Formula weight Anhydrous: 82.03 Trihydrate: 136.08 Assay Not less than 98.5% after drying DESCRIPTION Anhydrous: White, odourless, granular, hygroscopic powder Trihydrate: Colourless, transparent crystals or a granular crystalline powder, odourless or with a faint, acetic odour. Effloresces in warm, dry air. FUNCTIONAL USES Buffer CHARACTERISTICS IDENTIFICATION Solubility (Vol. 4) Very soluble in water; soluble in ethanol pH (Vol. 4) 8.0 - 9.5 (1 in 100 soln) Test for sodium (Vol. 4) Passes test Test for acetate (Vol. 4) Passes test Heat test Anhydrous: When heating the sample slowly, it first fuses gradually and boils, and later decomposes evolving an unpleasant odour of acetone. A solution of the residue gives alkaline reaction with litmus paper. Trihydrate: When heating the sample slowly, it liquefies. Then water evaporates, and a powder forms. By heating more strongly, the powder fuses, and becomes lumpy and later decomposes evolving an odour of acetone. A solution of the residue gives alkaline reaction with litmus paper. PURITY Loss on drying (Vol. 4) Anhydrous: Not more than 2.0% (120o, 4 h) Trihydrate: Between 36 and 42% (120o, 4 h) Test for potassium Negative test (Vol. -

Ammonium Acetate
Right to Know Hazardous Substance Fact Sheet Common Name: AMMONIUM ACETATE Synonyms: None CAS Number: 631-61-8 Chemical Name: Acetic Acid, Ammonium Salt RTK Substance Number: 0085 Date: April 2002 Revision: March 2011 DOT Number: UN 9079 Description and Use EMERGENCY RESPONDERS >>>> SEE LAST PAGE Ammonium Acetate is a white, crystalline (sand-like) solid Hazard Summary with a slight vinegar-like odor. It is used in chemical analysis, Hazard Rating NJDOH NFPA textile dyeing, and preserving meats. HEALTH 2 - FLAMMABILITY 1 - REACTIVITY 0 - POISONOUS GASES ARE PRODUCED IN FIRE Reasons for Citation f Ammonium Acetate is on the Right to Know Hazardous Hazard Rating Key: 0=minimal; 1=slight; 2=moderate; 3=serious; 4=severe Substance List because it is cited by DOT and IRIS. f Ammonium Acetate can affect you when inhaled. f Contact can irritate and burn the skin and eyes. f Inhaling Ammonium Acetate can irritate the nose, throat and lungs causing coughing, wheezing and/or shortness of breath. SEE GLOSSARY ON PAGE 5. FIRST AID Eye Contact Workplace Exposure Limits f Immediately flush with large amounts of water for at least 30 No occupational exposure limits have been established for minutes, lifting upper and lower lids. Remove contact Ammonium Acetate. However, it may pose a health risk. lenses, if worn, while flushing. Seek medical attention. Always follow safe work practices. Skin Contact f Quickly remove contaminated clothing. Immediately wash contaminated skin with large amounts of water. Inhalation f Remove the person from exposure. f Begin rescue breathing (using universal precautions) if breathing has stopped and CPR if heart action has stopped. -

United States Patent Office Patented Sept
3,274,105 United States Patent Office Patented Sept. 20, 1966 2 Such as carbon disulfide and percaptains). Nevertheless, 3,274,05 Within the above-mentioned limits, it is advisable to FIRE EXTENGUISHENG COMPOSITION choose the concentration of the solution according to the Norbert Mevel, Antony, France, assignor to Societe d’Etudes Chimiques poeir distrie et l'Agriculare nature of the fires to be dealt with: for fires of high boil S.E.C.P.I.A., Paris, France ing hydrocarbons and those of a large number of chem No Drawing. Filed July 5, 1963, Ser. No. 295,213 icals Such as alcohol, aldehydes, amines, petroleum ether, Claims priority, application France, Aag. 3, 1962, ethyl ether, etc., solutions containing from 300 to 400 905,979, Patent 1,338,454 g./l. of potassium acetate give the best results. But for 10 Clains. (C. 252-2) fires of low boiling hydrocarbons such as gasoline, solu O tions containing from about 500 to 600 g./l. are more This invention relates to fire extinguishing Solutions for effective to obtain complete extinction. use against dry fires and fires particularly difficult to ex It has also been found that by adding tetrapotassium tinguish such as those of hydrocarbons or inflammable pyrophosphate to acqueous solutions of potassium acetate, chemicals and mixed fires, that is to say, fires of con the extinguishing effect is considerably increased and that bustible solid substances (wood, paper, rags, etc.) soaked 5 the same results are obtained with a lower acetate con for example with hydrocarbons or chemicals. Centration. For example, a solution containing 300 g./I.