White Paper for a Honey Bee Genome Project the Honey Bee Genome Sequencing Consortiuma
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Genome Sequence of the Progenitor of the Wheat D Genome Aegilops Tauschii Ming-Cheng Luo1*, Yong Q
OPEN LETTER doi:10.1038/nature24486 Genome sequence of the progenitor of the wheat D genome Aegilops tauschii Ming-Cheng Luo1*, Yong Q. Gu2*, Daniela Puiu3*, Hao Wang4,5,6*, Sven O. Twardziok7*, Karin R. Deal1, Naxin Huo1,2, Tingting Zhu1, Le Wang1, Yi Wang1,2, Patrick E. McGuire1, Shuyang Liu1, Hai Long1, Ramesh K. Ramasamy1, Juan C. Rodriguez1, Sonny L. Van1, Luxia Yuan1, Zhenzhong Wang1,8, Zhiqiang Xia1, Lichan Xiao1, Olin D. Anderson2, Shuhong Ouyang2,8, Yong Liang2,8, Aleksey V. Zimin3, Geo Pertea3, Peng Qi4,5, Jeffrey L. Bennetzen6, Xiongtao Dai9, Matthew W. Dawson9, Hans-Georg Müller9, Karl Kugler7, Lorena Rivarola-Duarte7, Manuel Spannagl7, Klaus F. X. Mayer7,10, Fu-Hao Lu11, Michael W. Bevan11, Philippe Leroy12, Pingchuan Li13, Frank M. You13, Qixin Sun8, Zhiyong Liu8, Eric Lyons14, Thomas Wicker15, Steven L. Salzberg3,16, Katrien M. Devos4,5 & Jan Dvořák1 Aegilops tauschii is the diploid progenitor of the D genome of We conclude therefore that the size of the Ae. tauschii genome is about hexaploid wheat1 (Triticum aestivum, genomes AABBDD) and 4.3 Gb. an important genetic resource for wheat2–4. The large size and To assess the accuracy of our assembly, sequences of 195 inde- highly repetitive nature of the Ae. tauschii genome has until now pendently sequenced and assembled AL8/78 BAC clones8, which precluded the development of a reference-quality genome sequence5. contained 25,540,177 bp in 2,405 unordered contigs, were aligned to Here we use an array of advanced technologies, including ordered- Aet v3.0. Five contigs failed to align and six extended partly into gaps, clone genome sequencing, whole-genome shotgun sequencing, accounting for 0.25% of the total length of the contigs. -

Genomes:Genomes: Whatwhat Wewe Knowknow …… Andand Whatwhat Wewe Don’Tdon’T Knowknow
Genomes:Genomes: WhatWhat wewe knowknow …… andand whatwhat wewe don’tdon’t knowknow Complete draft sequence 2001 OctoberOctober 15,15, 20072007 Dr.Dr. StefanStefan Maas,Maas, BioSBioS Lehigh Lehigh U.U. © SMaas 2007 What we know Raw genome data © SMaas 2007 The range of genome sizes in the animal & plant kingdoms !! NoNo correlationcorrelation betweenbetween genomegenome sizesize andand complexitycomplexity © SMaas 2007 What accounts for the often massive and seemingly arbitrary differences in genome size observed among eukaryotic organisms? The fruit fly The mountain grasshopper Drosophila melanogaster Podisma pedestris 180 Mb 18,000 Mb The difference in genome size of a factor of 100 is difficult to explain in view of the apparently similar levels of evolutionary, developmental and behavioral complexity of these organisms. © SMaas 2007 ComplexityComplexity doesdoes notnot correlatecorrelate withwith genomegenome sizesize 3.4 × 10 9 bp 6.7 × 1011 bp Homo sapiens Amoeba dubia © SMaas 2007 ComplexityComplexity doesdoes notnot correlatecorrelate withwith genegene numbernumber ~31,000~31,000 genesgenes ~26,000~26,000 genesgenes ~50,000~50,000 genesgenes © SMaas 2007 IsIs anan ExpansionExpansion inin GeneGene NumberNumber drivingdriving EvolutionEvolution ofof HigherHigher Organisms?Organisms? Vertebrata 30,000 Urochordata 16,000 Arthropoda 14,000 Nematoda 21,000 Fungi 2,000 – 13,000 Vascular plants 25,000 – 60,000 Unicellular sps. 5,000 – 10,000 Prokaryotes 500 - 7,000 © SMaas 2007 Structure of DNA Watson and Crick in 1953 proposed that DNA is a double helix in which the 4 bases are base paired, Adenine (A) with Thymine (T) and Guanine (G) with Cytosine (C). © SMaas 2007 © SMaas 2007 © SMaas 2007 Steps in the folding of DNA to create an eukaryotic chromosome 30 nm fiber (6 nucleosomes per turn) FactorFactor ofof condensation:condensation: Ca.Ca. -

Saccharomyces Cerevisiae
letters to nature 19Department of Yeast Genetics, Institute of Molecular Medecine, John Radcliffe Hospital, Headington, Oxford OX3 9DU, UK The nucleotide sequence of 20L.N.C.I.B., Area Science Park, Padriciano 99, I-34012 Trieste, Italy 21GATC GmbH, Fitz-Arnold-Strasse 23, 78467 Konstanz, Germany Saccharomyces cerevisiae 22Carlsberg Laboratory, Gamle Carlsberg Vej 10, DK-2500 Copenhagen Valby, Denmark chromosome IV 23AGON GmbH, Glienicker Weg 185, D-12489 Berlin, Germany 24Katholieke Universiteit Leuven, Laboratory of Gene Technology, Willem de C. Jacq1, J. Alt-Mörbe2, B. Andre3, W. Arnold4, A. Bahr5, Croylaan, 42, B-3001 Leuven, Belgium J. P. G. Ballesta6, M. Bargues7, L. Baron8, A. Becker4, N. Biteau8, 25The Sanger Centre, Wellcome Trust Genome Campus, Hinxton, Cambridge H. Blöcker9, C. Blugeon1, J. Boskovic6, P. Brandt9, M. Brückner10 , CB10 1SA, UK M. J. Buitrago11 , F. Coster12, T. Delaveau1, F. del Rey11 , B. Dujon13, 26Department of Biochemistry, Stanford University, Beckman Center, Stanford L. G. Eide14, J. M. Garcia-Cantalejo6, A. Goffeau12, A. Gomez-Peris15, CA 94305-5307, USA C. Granotier8, V. Hanemann16, T. Hankeln5, J. D. Hoheisel17, W. Jäger9, 27The Genome Sequencing Center, Department of Genetics, Washington University, A. Jimenez6, J.-L. Jonniaux12, C. Krämer5, H. Küster4, P. Laamanen18, School of Medicine, 630 S. Euclid Avenue, St Louis, Missouri 63110, USA Y. Legros8, E. Louis19, S. Möller-Rieker5, A. Monnet8, M. Moro20, 28Martinsrieder Institut für Protein Sequenzen, Max-Planck-Institut für S. Müller-Auer10 , B. Nußbaumer4, N. Paricio7, L. Paulin18, J. Perea1, Biochemie, D-82152 Martinsried bei München, Germany. M. Perez-Alonso7, J. E. Perez-Ortin15, T. M. Pohl21, H. Prydz14, B. -
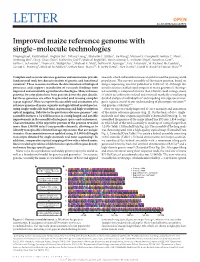
Improved Maize Reference Genome with Single-Molecule Technologies Yinping Jiao1, Paul Peluso2, Jinghua Shi3, Tiffany Liang3, Michelle C
OPEN LETTER doi:10.1038/nature22971 Improved maize reference genome with single-molecule technologies Yinping Jiao1, Paul Peluso2, Jinghua Shi3, Tiffany Liang3, Michelle C. Stitzer4, Bo Wang1, Michael S. Campbell1, Joshua C. Stein1, Xuehong Wei1, Chen-Shan Chin2, Katherine Guill5, Michael Regulski1, Sunita Kumari1, Andrew Olson1, Jonathan Gent6, Kevin L. Schneider7, Thomas K. Wolfgruber7, Michael R. May8, Nathan M. Springer9, Eric Antoniou1, W. Richard McCombie1, Gernot G. Presting7, Michael McMullen5, Jeffrey Ross-Ibarra10, R. Kelly Dawe6, Alex Hastie3, David R. Rank2 & Doreen Ware1,11 Complete and accurate reference genomes and annotations provide research, which will enable increases in yield to feed the growing world fundamental tools for characterization of genetic and functional population. The current assembly of the maize genome, based on variation1. These resources facilitate the determination of biological Sanger sequencing, was first published in 2009 (ref. 3). Although this processes and support translation of research findings into initial reference enabled rapid progress in maize genomics1, the origi- improved and sustainable agricultural technologies. Many reference nal assembly is composed of more than 100,000 small contigs, many genomes for crop plants have been generated over the past decade, of which are arbitrarily ordered and oriented, markedly complicating but these genomes are often fragmented and missing complex detailed analysis of individual loci6 and impeding investigation of inter- repeat regions2. Here we report the assembly and annotation of a genic regions crucial to our understanding of phenotypic variation7,8 reference genome of maize, a genetic and agricultural model species, and genome evolution9,10. using single-molecule real-time sequencing and high-resolution Here we report a vastly improved de novo assembly and annotation optical mapping. -

Apis Mellifera)
COMPUTATIONAL ANALYSIS OF DIFFICULT-TO-PREDICT GENES AND DETECTION OF LINEAGE-SPECIFIC GENES IN HONEY BEE (APIS MELLIFERA) A Dissertation submitted to the Faculty of the Graduate School of Arts and Sciences of Georgetown University in partial fulfillment of the requirements for the degree of Doctor of Philosophy in Biology. By Anna K. Bennett, M.S. Washington, DC July 29, 2013 Copyright 2013 by Anna K. Bennett All Rights Reserved ii COMPUTATIONAL ANALYSIS OF DIFFICULT-TO-PREDICT GENES AND DETECTION OF LINEAGE-SPECIFIC GENES IN HONEY BEE (APIS MELLIFERA) Anna K. Bennett, M.S. Thesis Advisor: Christine G. Elsik, Ph.D. ABSTRACT Honey bees are key agricultural pollinators and a research model for social behavior and the evolution of eusociality. Generation of reliable gene predictions is critical to the success of laboratory experiments and comparative analyses for sequenced genomes. The honey bee genome, published in 2006 by the Honey Bee Genome Sequencing Consortium, had fewer predicted genes than expected, partially due to a lack of transcriptome data and protein homologs from closely related species. As part of ongoing Consortium efforts, genomes of two closely related taxa were sequenced, dwarf honey bee (Apis florea) and buff-tailed bumble bee (Bombus terrestris), the draft A. mellifera genome was improved with additional sequence, and multiple A. mellifera tissue transcriptomes were sequenced. The Consortium predicted genes using seven methods and used these data to produce an improved official gene set (OGSv3.2) with ~5000 more protein-coding genes than the first set (OGSv1.0). We present our approach to detect previously unknown genes in A. -

Genome-Wide Analysis of Admixture and Adaptation in the Africanized Honeybee
Nelson, R. M., Wallberg, A., Simões, Z. L. P., Lawson, D. J., & Webster, M. T. (2017). Genome-wide analysis of admixture and adaptation in the Africanized honeybee. Molecular Ecology, 26(14), 3603-3617. https://doi.org/10.1111/mec.14122 Peer reviewed version License (if available): CC BY-NC Link to published version (if available): 10.1111/mec.14122 Link to publication record in Explore Bristol Research PDF-document This is the author accepted manuscript (AAM). The final published version (version of record) is available online via Wiley at https://onlinelibrary.wiley.com/doi/abs/10.1111/mec.14122 . Please refer to any applicable terms of use of the publisher. University of Bristol - Explore Bristol Research General rights This document is made available in accordance with publisher policies. Please cite only the published version using the reference above. Full terms of use are available: http://www.bristol.ac.uk/red/research-policy/pure/user-guides/ebr-terms/ Genome-wide analysis of admixture and adaptation in the Africanized honeybee Ronald M. Nelson1, Andreas Wallberg1, Zilá Luz Paulino Simões2, Daniel J. Lawson3, Matthew T. Webster1* 1. Department of Medical Biochemistry and Microbiology, Science for Life Laboratory, Uppsala University, Uppsala, Sweden. 2. Department of Biology, University of São Paulo, São Paulo, Brazil. 3. Department of Mathematics, University of Bristol, Bristol, United Kingdom. Keywords: Africanized honeybee, admixture, introgression, adaptation, biological invasion, natural selection *[email protected] Running title: Adaptation in Africanized honeybees 1 Abstract Genetic exchange by hybridization or admixture can make an important contribution to evolution, and introgression of favourable alleles can facilitate adaptation to new environments. -

Recombination in Diverse Maize Is Stable, Predictable, and Associated with Genetic Load
Recombination in diverse maize is stable, predictable, and associated with genetic load Eli Rodgers-Melnicka,1, Peter J. Bradburya,b,1, Robert J. Elshirea, Jeffrey C. Glaubitza, Charlotte B. Acharyaa, Sharon E. Mitchella, Chunhui Lic, Yongxiang Lic, and Edward S. Bucklera,b aInstitute for Genomic Diversity, Cornell University, Ithaca, NY 14853; bUS Department of Agriculture-Agricultural Research Service, Ithaca, NY 14853; and cInstitute of Crop Science, Chinese Academy of Agricultural Sciences, Beijing 100081, China Edited by Qifa Zhang, Huazhong Agricultural University, Wuhan, China, and approved February 6, 2015 (received for review July 21, 2014) Among the fundamental evolutionary forces, recombination ar- On a molecular level, chromatin structure heavily influences guably has the largest impact on the practical work of plant the cross-over rate in plants. Not only are heterochromatic breeders. Varying over 1,000-fold across the maize genome, the regions generally depleted of cross-overs (11), but KO of cytosine- local meiotic recombination rate limits the resolving power of DNA-methyl-transferase (MET1)inArabidopsis thaliana leads quantitative trait mapping and the precision of favorable allele to both genome-wide CpG hypomethylation and a relative in- introgression. The consequences of low recombination also theo- crease in the proportion of cross-overs within the euchromatic – retically extend to the species-wide scale by decreasing the power chromosomal arms (12 14). Nucleotide content may also be as- of selection relative to genetic drift, and thereby hindering the sociated with the local frequency of recombination, potentially purging of deleterious mutations. In this study, we used genotyp- due to the effect of GC-biased gene conversion (bGC) during res- ing-by-sequencing (GBS) to identify 136,000 recombination break- olution of heteroduplexes that form at cross-over junctions (15). -

Co-Expression of Neighboring Genes in the Zebrafish (Danio Rerio) Genome
Int. J. Mol. Sci. 2009, 10, 3658-3670; doi:10.3390/ijms10083658 OPEN ACCESS International Journal of Molecular Sciences ISSN 1422-0067 www.mdpi.com/journal/ijms Article Co-Expression of Neighboring Genes in the Zebrafish (Danio rerio) Genome Huai-Kuang Tsai 1, Pei-Ying Huang 2, Cheng-Yan Kao 2 and Daryi Wang 3,* 1 Institute of Information Science, Academia Sinica, 128 Sec. 2, Academia Rd, Nankang, 115, Taipei, Taiwan; E-Mail: [email protected] (H.-K.T.) 2 Department of Computer Science and Information Engineering, National Taiwan University, Taipei 106, Taiwan; E-Mails: [email protected] (P.-Y.H.); [email protected] (C.-Y.K.) 3 Biodiversity Research Center, Academia Sinica, 128 Sec. 2, Academia Rd, Nankang, 115, Taipei, Taiwan * Author to whom correspondence should be addressed; E-Mail: [email protected] (D.W.); Tel. +886-2-27890159; Fax: +886-2-27829624. Received: 14 July 2009; in revised form: 11 August 2009 / Accepted: 20 August 2009 / Published: 21 August 2009 Abstract: Neighboring genes in the eukaryotic genome have a tendency to express concurrently, and the proximity of two adjacent genes is often considered a possible explanation for their co-expression behavior. However, the actual contribution of the physical distance between two genes to their co-expression behavior has yet to be defined. To further investigate this issue, we studied the co-expression of neighboring genes in zebrafish, which has a compact genome and has experienced a whole genome duplication event. Our analysis shows that the proportion of highly co-expressed neighboring pairs (Pearson’s correlation coefficient R>0.7) is low (0.24% ~ 0.67%); however, it is still significantly higher than that of random pairs. -

Ceranae, an Emergent Pathogen of Honey Bees
Genomic Analyses of the Microsporidian Nosema ceranae, an Emergent Pathogen of Honey Bees R. Scott Cornman1, Yan Ping Chen1, Michael C. Schatz2, Craig Street3, Yan Zhao4, Brian Desany5, Michael Egholm5, Stephen Hutchison5, Jeffery S. Pettis1, W. Ian Lipkin3, Jay D. Evans1* 1 USDA-ARS Bee Research Lab, Beltsville, Maryland, United States of America, 2 Center for Bioinformatics and Computational Biology, University of Maryland, College Park, Maryland, United States of America, 3 Center for Infection and Immunity, Mailman School of Public Health, Columbia University, New York, New York, United States of America, 4 USDA-ARS Molecular Plant Pathology Laboratory, Beltsville, Maryland, United States of America, 5 454 Life Sciences/Roche Applied Sciences, Branford, Connecticut, United States of America Abstract Recent steep declines in honey bee health have severely impacted the beekeeping industry, presenting new risks for agricultural commodities that depend on insect pollination. Honey bee declines could reflect increased pressures from parasites and pathogens. The incidence of the microsporidian pathogen Nosema ceranae has increased significantly in the past decade. Here we present a draft assembly (7.86 MB) of the N. ceranae genome derived from pyrosequence data, including initial gene models and genomic comparisons with other members of this highly derived fungal lineage. N. ceranae has a strongly AT-biased genome (74% A+T) and a diversity of repetitive elements, complicating the assembly. Of 2,614 predicted protein-coding sequences, we conservatively estimate that 1,366 have homologs in the microsporidian Encephalitozoon cuniculi, the most closely related published genome sequence. We identify genes conserved among microsporidia that lack clear homology outside this group, which are of special interest as potential virulence factors in this group of obligate parasites. -

Genome-Wide Analysis of Local Chromatin Packing in Arabidopsis Thaliana
Downloaded from genome.cshlp.org on September 24, 2021 - Published by Cold Spring Harbor Laboratory Press Research Genome-wide analysis of local chromatin packing in Arabidopsis thaliana Congmao Wang,1,3 Chang Liu,1,3 Damian Roqueiro,2 Dominik Grimm,2 Rebecca Schwab,1 Claude Becker,1 Christa Lanz,1 and Detlef Weigel1 1Department of Molecular Biology, Max Planck Institute for Developmental Biology, 72076 Tubingen,€ Germany; 2Machine Learning and Computational Biology Research Group, Max Planck Institute for Developmental Biology and Max Planck Institute for Intelligent Systems, 72076 Tubingen,€ Germany The spatial arrangement of interphase chromosomes in the nucleus is important for gene expression and genome function in animals and in plants. The recently developed Hi-C technology is an efficacious method to investigate genome packing. Here we present a detailed Hi-C map of the three-dimensional genome organization of the plant Arabidopsis thaliana. We find that local chromatin packing differs from the patterns seen in animals, with kilobasepair-sized segments that have much higher intrachromosome interaction rates than neighboring regions, representing a dominant local structural feature of genome conformation in A. thaliana. These regions, which appear as positive strips on two-dimensional representations of chromatin interaction, are enriched in epigenetic marks H3K27me3, H3.1, and H3.3. We also identify more than 400 insulator-like regions. Furthermore, although topologically associating domains (TADs), which are prominent in animals, are not an obvious feature of A. thaliana genome packing, we found more than 1000 regions that have properties of TAD boundaries, and a similar number of regions analogous to the interior of TADs. -

Genome-Wide DNA Alterations in X-Irradiated Human Gingiva Fibroblasts
International Journal of Molecular Sciences Article Genome-Wide DNA Alterations in X-Irradiated Human Gingiva Fibroblasts 1,2, 1, 1 1,2 1 Neetika Nath y , Lisa Hagenau y , Stefan Weiss , Ana Tzvetkova , Lars R. Jensen , Lars Kaderali 2 , Matthias Port 3, Harry Scherthan 3 and Andreas W. Kuss 1,* 1 Human Molecular Genetics Group, Department of Functional Genomics, Interfaculty Institute for Genetics and Functional Genomics, University Medicine Greifswald, 17475 Greifswald, Germany; [email protected] (N.N.); [email protected] (L.H.); [email protected] (S.W.); [email protected] (A.T.); [email protected] (L.R.J.) 2 Institute of Bioinformatics, University Medicine Greifswald, 17475 Greifswald, Germany; [email protected] 3 Bundeswehr Institute for Radiobiology Affiliated to the University of Ulm, 80937 München, Germany; [email protected] (M.P.); [email protected] (H.S.) * Correspondence: [email protected]; Tel.: +49-3834-420-5814 These authors contributed equally. y Received: 8 July 2020; Accepted: 31 July 2020; Published: 12 August 2020 Abstract: While ionizing radiation (IR) is a powerful tool in medical diagnostics, nuclear medicine, and radiology, it also is a serious threat to the integrity of genetic material. Mutagenic effects of IR to the human genome have long been the subject of research, yet still comparatively little is known about the genome-wide effects of IR exposure on the DNA-sequence level. In this study, we employed high throughput sequencing technologies to investigate IR-induced DNA alterations in human gingiva fibroblasts (HGF) that were acutely exposed to 0.5, 2, and 10 Gy of 240 kV X-radiation followed by repair times of 16 h or 7 days before whole-genome sequencing (WGS). -

Saccharomyces Cerevisiae HOWARD BUSSEY*T, DAVID B
Proc. Natl. Acad. Sci. USA Vol. 92, pp. 3809-3813, April 1995 Genetics The nucleotide sequence of chromosome I from Saccharomyces cerevisiae HOWARD BUSSEY*t, DAVID B. KABACKI, WUWEI ZHONG*, DAHN T. Vo*, MICHAEL W. CLARK*, NATHALIE FORTIN*, JOHN HALL*, B. F. FRANCIs OUELLETTE*, TERESA KENG§, ARNOLD B. BARTONI, YUPING SUt, CHRIS J. DAVIESt, AND REG K. STORMS*II *Yeast Chromosome I Project, Biology Department, McGill University, Montreal, QC, Canada H3A lB1; *Department of Microbiology and Molecular Genetics, University of Medicine and Dentistry of New Jersey-New Jersey Medical School, Newark, NJ 07103; §Department of Microbiology and Immunology, McGill University, Montreal, QC, Canada H3A 2B4; 1Department of Biology, University of North Carolina, Chapel Hill, NC 27599; and I1Biology Department, Concordia University, Montreal, QC, Canada H3G 1M8 Communicated by Phillips W Robbins, Massachusetts Institute of Technology, Cambridge, MA, January 12, 1995 ABSTRACT Chromosome I from the yeast Saccharomyces plates for DNA sequencing. These were the library of Riles et cerevisiae contains a DNA molecule of -231 kbp and is the aL (8), a cosmid from the collection of Dujon (9), chromosome smallest naturally occurring functional eukaryotic nuclear walking (10), and PCR amplified fragments of genomic DNA. chromosome so far characterized. The nucleotide sequence of DNA fragments, except those generated by PCR which were this chromosome has been determined as part of an interna- used directly, were subcloned into the Bluescript KS(+) plas- tional collaboration to sequence the entire yeast genome. The mid from Stratagene prior to sequencing. All DNA sequencing chromosome contains 89 open reading frames and 4 tRNA was performed using double-stranded DNA templates.