A Dissertation Entitled Hypothalamic Melanocortin 4 Receptors Regulate
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

1 Evidence for Gliadin Antibodies As Causative Agents in Schizophrenia
1 Evidence for gliadin antibodies as causative agents in schizophrenia. C.J.Carter PolygenicPathways, 20 Upper Maze Hill, Saint-Leonard’s on Sea, East Sussex, TN37 0LG [email protected] Tel: 0044 (0)1424 422201 I have no fax Abstract Antibodies to gliadin, a component of gluten, have frequently been reported in schizophrenia patients, and in some cases remission has been noted following the instigation of a gluten free diet. Gliadin is a highly immunogenic protein, and B cell epitopes along its entire immunogenic length are homologous to the products of numerous proteins relevant to schizophrenia (p = 0.012 to 3e-25). These include members of the DISC1 interactome, of glutamate, dopamine and neuregulin signalling networks, and of pathways involved in plasticity, dendritic growth or myelination. Antibodies to gliadin are likely to cross react with these key proteins, as has already been observed with synapsin 1 and calreticulin. Gliadin may thus be a causative agent in schizophrenia, under certain genetic and immunological conditions, producing its effects via antibody mediated knockdown of multiple proteins relevant to the disease process. Because of such homology, an autoimmune response may be sustained by the human antigens that resemble gliadin itself, a scenario supported by many reports of immune activation both in the brain and in lymphocytes in schizophrenia. Gluten free diets and removal of such antibodies may be of therapeutic benefit in certain cases of schizophrenia. 2 Introduction A number of studies from China, Norway, and the USA have reported the presence of gliadin antibodies in schizophrenia 1-5. Gliadin is a component of gluten, intolerance to which is implicated in coeliac disease 6. -

A Computational Approach for Defining a Signature of Β-Cell Golgi Stress in Diabetes Mellitus
Page 1 of 781 Diabetes A Computational Approach for Defining a Signature of β-Cell Golgi Stress in Diabetes Mellitus Robert N. Bone1,6,7, Olufunmilola Oyebamiji2, Sayali Talware2, Sharmila Selvaraj2, Preethi Krishnan3,6, Farooq Syed1,6,7, Huanmei Wu2, Carmella Evans-Molina 1,3,4,5,6,7,8* Departments of 1Pediatrics, 3Medicine, 4Anatomy, Cell Biology & Physiology, 5Biochemistry & Molecular Biology, the 6Center for Diabetes & Metabolic Diseases, and the 7Herman B. Wells Center for Pediatric Research, Indiana University School of Medicine, Indianapolis, IN 46202; 2Department of BioHealth Informatics, Indiana University-Purdue University Indianapolis, Indianapolis, IN, 46202; 8Roudebush VA Medical Center, Indianapolis, IN 46202. *Corresponding Author(s): Carmella Evans-Molina, MD, PhD ([email protected]) Indiana University School of Medicine, 635 Barnhill Drive, MS 2031A, Indianapolis, IN 46202, Telephone: (317) 274-4145, Fax (317) 274-4107 Running Title: Golgi Stress Response in Diabetes Word Count: 4358 Number of Figures: 6 Keywords: Golgi apparatus stress, Islets, β cell, Type 1 diabetes, Type 2 diabetes 1 Diabetes Publish Ahead of Print, published online August 20, 2020 Diabetes Page 2 of 781 ABSTRACT The Golgi apparatus (GA) is an important site of insulin processing and granule maturation, but whether GA organelle dysfunction and GA stress are present in the diabetic β-cell has not been tested. We utilized an informatics-based approach to develop a transcriptional signature of β-cell GA stress using existing RNA sequencing and microarray datasets generated using human islets from donors with diabetes and islets where type 1(T1D) and type 2 diabetes (T2D) had been modeled ex vivo. To narrow our results to GA-specific genes, we applied a filter set of 1,030 genes accepted as GA associated. -

User's Manual Mobile Phone
User’s Manual Mobile Phone Model: AM206 FCC ID: UOSAM206 1 TABLE OF CONTENT 1. BASIC INFORMATION .................... 6 1.1. BRIEF INTRODUCTION ........................................... 6 2. BEFORE USE ..................................... 7 2.1. NAME AND EXPLANATION OF EACH PART ............. 7 2.1.1. Appearance Sketch Map ........................... 7 2.1.2. Description of the Standby Pictures .......... 7 2.1.3. Description of Keys .................................. 8 2.2. INSTALL SIM CARD AND MEMORY CARD ........... 10 2.2.1. SIM Card ................................................. 10 2.2.2. Inserting and Taking out SIM Card ........ 11 2.2.3. Memory Card .......................................... 12 2.3. BATTERY ............................................................ 12 2.3.1. Install the Battery .................................... 12 2.3.2. Charging .................................................. 13 2.4. SECURITY PASSWORD ......................................... 14 2 3. QUICK USER GUIDE ..................... 15 3.1. DIAL A CALL ....................................................... 15 3.2. REJECT A CALL ................................................... 15 3.3. RECEIVE A CALL ................................................. 15 3.4. END A CALL ........................................................ 16 3.5. EMERGENCY SERVICES ....................................... 16 3.6. EXTENSION SPEED DIAL ..................................... 16 3.7. DIALED CALLS/MISSED CALLS/RECEIVED CALLS/REJECTED CALLS ............................................. -

Uses of Bremelanotide in Therapy for Female Sexual
(19) TZZ __T (11) EP 2 916 856 B1 (12) EUROPEAN PATENT SPECIFICATION (45) Date of publication and mention (51) Int Cl.: of the grant of the patent: A61K 9/00 (2006.01) A61K 38/12 (2006.01) 19.09.2018 Bulletin 2018/38 A61P 15/00 (2006.01) C07K 7/64 (2006.01) (21) Application number: 13851014.4 (86) International application number: PCT/US2013/068386 (22) Date of filing: 05.11.2013 (87) International publication number: WO 2014/071339 (08.05.2014 Gazette 2014/19) (54) USES OF BREMELANOTIDE IN THERAPY FOR FEMALE SEXUAL DYSFUNCTION VERWENDUNG VON BREMELANOTID IN DER THERAPIE VON WEIBLICHER SEXUELLER DYSFUNKTION UTILISATIONS DE BRÉMÉLANOTIDE DANS UNE THÉRAPIE DE DYSFONCTIONNEMENT SEXUEL FÉMININ (84) Designated Contracting States: • "Palatin Technologies Presents Positive Results AL AT BE BG CH CY CZ DE DK EE ES FI FR GB for Phase 2B Bremelanotide Female Sexual GR HR HU IE IS IT LI LT LU LV MC MK MT NL NO Dysfunction Trial", PRNewswire , 8 November PL PT RO RS SE SI SK SM TR 2012 (2012-11-08), XP002757483, Retrieved from the Internet: (30) Priority: 05.11.2012 US 201261722511 P URL:http://www.palatin.com/investors/press 28.02.2013 US 201361770535 P -releases/ [retrieved on 2016-05-09] • ROSEN R C ET AL: "Evaluation of the safety, (43) Date of publication of application: pharmacokineticsand pharmacodynamic effects 16.09.2015 Bulletin 2015/38 of subcutaneously administered PT-141, a melanocortin receptor agonist, in healthy male (73) Proprietor: Palatin Technologies, Inc. subjects and in patients with an inadequate Cranbury NJ 08512 (US) response to Viagra(R)", INTERNATIONAL JOURNAL OF IMPOTENCE RESEARCH, (72) Inventors: STOCKTON, BASINGSTOKE, GB, vol. -

Supplementary Materials
Supplementary materials Supplementary Table S1: MGNC compound library Ingredien Molecule Caco- Mol ID MW AlogP OB (%) BBB DL FASA- HL t Name Name 2 shengdi MOL012254 campesterol 400.8 7.63 37.58 1.34 0.98 0.7 0.21 20.2 shengdi MOL000519 coniferin 314.4 3.16 31.11 0.42 -0.2 0.3 0.27 74.6 beta- shengdi MOL000359 414.8 8.08 36.91 1.32 0.99 0.8 0.23 20.2 sitosterol pachymic shengdi MOL000289 528.9 6.54 33.63 0.1 -0.6 0.8 0 9.27 acid Poricoic acid shengdi MOL000291 484.7 5.64 30.52 -0.08 -0.9 0.8 0 8.67 B Chrysanthem shengdi MOL004492 585 8.24 38.72 0.51 -1 0.6 0.3 17.5 axanthin 20- shengdi MOL011455 Hexadecano 418.6 1.91 32.7 -0.24 -0.4 0.7 0.29 104 ylingenol huanglian MOL001454 berberine 336.4 3.45 36.86 1.24 0.57 0.8 0.19 6.57 huanglian MOL013352 Obacunone 454.6 2.68 43.29 0.01 -0.4 0.8 0.31 -13 huanglian MOL002894 berberrubine 322.4 3.2 35.74 1.07 0.17 0.7 0.24 6.46 huanglian MOL002897 epiberberine 336.4 3.45 43.09 1.17 0.4 0.8 0.19 6.1 huanglian MOL002903 (R)-Canadine 339.4 3.4 55.37 1.04 0.57 0.8 0.2 6.41 huanglian MOL002904 Berlambine 351.4 2.49 36.68 0.97 0.17 0.8 0.28 7.33 Corchorosid huanglian MOL002907 404.6 1.34 105 -0.91 -1.3 0.8 0.29 6.68 e A_qt Magnogrand huanglian MOL000622 266.4 1.18 63.71 0.02 -0.2 0.2 0.3 3.17 iolide huanglian MOL000762 Palmidin A 510.5 4.52 35.36 -0.38 -1.5 0.7 0.39 33.2 huanglian MOL000785 palmatine 352.4 3.65 64.6 1.33 0.37 0.7 0.13 2.25 huanglian MOL000098 quercetin 302.3 1.5 46.43 0.05 -0.8 0.3 0.38 14.4 huanglian MOL001458 coptisine 320.3 3.25 30.67 1.21 0.32 0.9 0.26 9.33 huanglian MOL002668 Worenine -

Preclinical Effects of Melanocortins in Male Sexual Dysfunction
International Journal of Impotence Research (2008) 20, S11–S16 & 2008 Nature Publishing Group All rights reserved 0955-9930/08 $30.00 www.nature.com/ijir Preclinical effects of melanocortins in male sexual dysfunction AM Shadiack1 and S Althof2 1Locus Pharmaceuticals, Blue Bell, PA, USA and 2The Center for Marital and Sexual Health of South Florida, West Palm Beach, FL, USA The neurobiology of sexual behavior involves the interrelationships between sex steroids and neurotransmitters that result in both central nervous system (CNS) effects and effects in the genitalia. Tools such as positron emission tomography (PET) and functional magnetic resonance imaging (fMRI) scanning can help determine what areas of the brain are activated under sexual stimulation. Our understanding of the role of various neurotransmitters, neurosteroids and other CNS-acting compounds is improving. The role of CNS-acting compounds such as dopamine agonists in the treatment of male sexual dysfunction is under active investigation. Melanocortins have CNS and peripheral roles in a wide variety of bodily functions. The melanocortin agonist bremelanotide appears to act in the CNS to promote erections in preclinical models, and may also stimulate behaviors that facilitate sexual activity beyond their erectogenic effects. International Journal of Impotence Research (2008) 20, S11–S16; doi:10.1038/ijir.2008.17 Keywords: erectile dysfunction; neuroanatomy; neurophysiology; CNS-acting agents; melanocortins; bremelanotide Introduction Neuroanatomy of male sexual response The neurobiology of sexual behavior involves the Sex and the brain interrelationships between sex steroids and neuro- Sexual arousal can now be studied with such transmitters that result in both central nervous sophisticated tools as PET (positron emission system (CNS) effects and effects in the genitalia. -

Brain Nuclear Receptors and Body Weight Regulation
Brain nuclear receptors and body weight regulation Yong Xu, … , Bert W. O’Malley, Joel K. Elmquist J Clin Invest. 2017;127(4):1172-1180. https://doi.org/10.1172/JCI88891. Review Series Neural pathways, especially those in the hypothalamus, integrate multiple nutritional, hormonal, and neural signals, resulting in the coordinated control of body weight balance and glucose homeostasis. Nuclear receptors (NRs) sense changing levels of nutrients and hormones, and therefore play essential roles in the regulation of energy homeostasis. Understanding the role and the underlying mechanisms of NRs in the context of energy balance control may facilitate the identification of novel targets to treat obesity. Notably, NRs are abundantly expressed in the brain, and emerging evidence indicates that a number of these brain NRs regulate multiple aspects of energy balance, including feeding, energy expenditure and physical activity. In this Review we summarize some of the recent literature regarding effects of brain NRs on body weight regulation and discuss mechanisms underlying these effects. Find the latest version: http://jci.me/88891/pdf REVIEW SERIES: NUCLEAR RECEPTORS The Journal of Clinical Investigation Series Editor: Mitchell A. Lazar Brain nuclear receptors and body weight regulation Yong Xu,1,2 Bert W. O’Malley,2 and Joel K. Elmquist3 1Children’s Nutrition Research Center, Department of Pediatrics, and 2Department of Molecular and Cellular Biology, Baylor College of Medicine, Houston, Texas, USA. 3Division of Hypothalamic Research, Department of Internal Medicine, University of Texas Southwestern Medical Center, Dallas, Texas, USA. Neural pathways, especially those in the hypothalamus, integrate multiple nutritional, hormonal, and neural signals, resulting in the coordinated control of body weight balance and glucose homeostasis. -

Modi Dissertation 2012 Submitted To
Distribution Agreement In presenting this thesis or dissertation as a partial fulfillment of the requirements for an advanced degree from Emory University, I hereby grant to Emory University and its agents the non-exclusive license to archive, make accessible, and display my thesis or dissertation in whole or in part in all forms of media, now or hereafter known, including display on the world wide web. I understand that I may select some access restrictions as part of the online submission of this thesis or dissertation. I retain all ownership rights to the copyright of the thesis or dissertation. I also retain the right to use in future works (such as articles or books) all or part of this thesis or dissertation. Signature: ________________________ ______________________ Meera Modi Date Identifying Novel Therapeutic Strategies for Enhancing Social Cognition Using Functional Animal Models By Meera Modi Doctor of Philosophy Graduate Division of Biological and Biomedical Science Neuroscience _________________________________________ Larry Young, PhD Advisor _________________________________________ Lisa Parr, PhD Committee Member _________________________________________ Diana Robins, PhD Committee Member _________________________________________ Randy Hall, PhD Committee Member _________________________________________ Kerry J. Ressler, MD/PhD Committee Member Accepted: _________________________________________ Lisa A. Tedesco, Ph.D. Dean of the James T. Laney School of Graduate Studies ___________________ Date Identifying Novel Therapeutic -

Female Sexual Interest / Arousal Disorder
Female Sexual Interest / See SSRI sexual dysfunction Arousal Disorder decision support Asse ss for me dica tions w hich Refer to appropriate SSRI may affect sexual function History of physical/sexual/emotional abuse Present psychosocial services other (i.e SSRI, TCA, contraceptives, and/or intimate partner violence? and agencies available Benzodiazepines, antipsychotics) in community Reassess need for medication and transition if Substance use Appropriate Evaluate for partner Obtain full medical/sexual/ disorder? Present See corresponding Sexual dysfunction psychosocial history decision support Comorbid psychiatric Present disorder present? Treat underlyingPresent Evaluate for non-gynecological cause and reassess medical etiology Commorbidity absent Evaluate for gynecological etiology Symptom Is duration greater than 6 months Symptom persistence and causing significant distre ss? persistence Yes Female with Sexual Inte re st Arousa l Disorder Asse ss a nd e sta blish treatment expectations a nd goals Behavior Modification: Lifestyle changes Psychotherapy: Body image Sexual therapy Consider Sleep hygiene Trailored intervention Couples therapy combination therapy Stress reduction Based on H&P Mindfulness Pelvic therapy Relationship building Sexual novelty Inadequate response Continue Psychotherapy and Behavior modification interventions Inadequate response Consider topical oils (i.e. Zestra) in addition to Bupropion Pharmacotherapy Inadequate response Buspirone is less studied, however, it may be useful in some circumstances Postmenopausal? Buspirone No Yes Consider testosterone Flibanserin therapy. Consider consultation Inadequate response Bremelanotide FEMALE SEXUAL INTEREST /AROUSAL DISORDER Bupropion XL Antidepressant DOSING: starting dose 150mg XL qAM. Increase after 1 week to 300mg XL Wellbutrin XL (dopamine and qAM. Target dose 300mg- 450mg XL qAM. Max dose 450mg XL qAM. norepinephrine uptake inhibitor) SIDE EFFECTS: (common) insomnia, nausea, agitation, tremor (rare, serious) seizures, especially in patients with a history of seizures or eating disorders. -

Bremelanotide in HSDD Presented at the 22Nd Annual Fall Scientific Meeting of 1 2 2 2 3 Sexual Medicine Society of North America Anita H
222 The Neurobiology and Efficacy of Bremelanotide in HSDD Presented at the 22nd Annual Fall Scientific Meeting of 1 2 2 2 3 Sexual Medicine Society of North America Anita H. Clayton, Johna Lucas, Robert Jordan, Carl Spana, James G. Pfaus November 3– 6, 2016 Scottsdale, AZ 1University of Virginia, Charlottesville, VA, USA; 2Palatin Technologies, Cranbury, NJ, USA; 3Concordia University, Montréal, Québec, Canada • Introduction Mechanism of Sexual and Reward Circuitry Rat Study Human Study — Decreased (less distress) both the total score and • These neurochemicals act on similar regions of the brain, • BMT’s downstream CNS effects of increasing arousal • Among premenopausal women with HSDD with or scores on the desire, arousal, and orgasm items of • Female sexual dysfunctions are classified as dysfunctions Sexual Response such as the medial preoptic area (mPOA) in the hypothalamus, and desire are thought to result from its action as a without FSAD, subcutaneous (SC) administration of the FSDS–Desire-Arousal-Orgasm measure. Mean of desire (eg, hypoactive sexual desire disorder [HSDD]), attention- and reward-related regions of the limbic system, MC-receptor agonist BMT 1.25 or 1.75 mg: (SE) change, –11.1 (12.0) vs –6.8 (13.6); BMT vs arousal (eg, female sexual arousal disorder [FSAD]), • Excitatory signals are regulated by dopamine (DA), placebo, respectively ( P=0.0014; Figure 7 )11 and the prefrontal cortex • Among female rats primed with estrogen and proges - — Significantly increased the number of sexually satisfying delay or absence of orgasm, or sexual pain (dyspareunia norepinephrine (NE), oxytocin, and the melanocortins 5,6 — Gamma aminobutyric acid projections help regulate DA terone or estrogen alone, BMT significantly increased events (SSEs) vs placebo when taken 45 minutes or vaginismus) 1 (MCs) Figure 7. -

Conversations with Women About Female Sexual Dysfunction (FSD)
235 Conversations With Women About Female Sexual Dysfunction (FSD) and Treatment With Bremelanotide Presented at the 22nd Annual Fall Scientific Meeting of 1 2 3 4 5 5 6 Sexual Medicine Society of North America Patricia E. Koochaki , Stanley Althof , Sheryl A. Kingsberg , Michael A. Perelman , Johna Lucas , Robert Jordan , Dennis A. Revicki November 3– 6, 2016 Scottsdale, AZ 1PEK Market Research, Inc., Cincinnati, OH, USA; 2Case Western Reserve University School of Medicine, Cleveland, OH, USA; 3University Hospitals Case Medical Center, Cleveland, OH, USA; 4Weill Cornell Medical College, New York, NY, USA; 5Palatin Technologies, Cranbury, NJ, USA; Evidera, Bethesda, MD, USA • Recognizing There Was a Problem Partner and Relationship Effects Physical Effects of BMT Sexual Satisfaction Increased Summary of Benefits That Women With HSDD With/Without Introduction Results Decreased Arousal During the interviews, women described how they Partner Blamed Himself • About three-quarters of all participants reported experiencing Approximately two-thirds of participants taking 1.25 and 1.75 mg Female sexual dysfunctions (FSD) include a Pre-Study: Conceptualizing and Explaining Why began to recognize they were experiencing sexual “There were a couple of times where he did blame himself. He physical effects from BMT of BMT reported an improvement in sexual satisfaction. range of distressing, multifactorial conditions, They Experienced FSD difficulties that required intervention. thought something was wrong with him.” • Nine of 10 women taking the 1.75-mg dose of BMT reported “A positive way …I think it restored or rebuilt what [sexual such as dysfunctions of sexual interest/desire, • Most women were unaware that FSD is a recognized “Because for a young man our age, he’s thinking like, it must be me. -
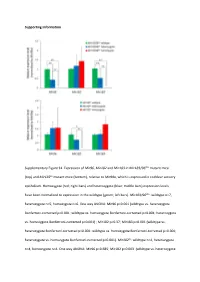
Supporting Information Supplementary Figure S1
Supporting Information Supplementary Figure S1. Expression of Mir96, Mir182 and Mir183 in Mir183/96dko mutant mice (top) and Mir182ko mutant mice (bottom), relative to Mir99a, which is expressed in cochlear sensory epithelium. Homozygote (red; right bars) and heterozygote (blue; middle bars) expression levels have been normalised to expression in the wildtype (green; left bars). Mir183/96dko: wildtype n=7, heterozygote n=5, homozygote n=6. One way ANOVA: Mir96 p<0.001 (wildtype vs. heterozygote Bonferroni‐corrected p<0.001; wildtype vs. homozygote Bonferroni‐corrected p<0.001; heterozygote vs. homozygote Bonferroni‐corrected p=0.001) ; Mir182 p=0.37; Mir183 p<0.001 (wildtype vs. heterozygote Bonferroni‐corrected p=0.001; wildtype vs. homozygote Bonferroni‐corrected p<0.001; heterozygote vs. homozygote Bonferroni‐corrected p<0.001). Mir182ko: wildtype n=4, heterozygote n=4, homozygote n=4. One way ANOVA: Mir96 p=0.685; Mir182 p=0.003 (wildtype vs. heterozygote Bonferroni‐corrected p=0.397; wildtype vs. homozygote Bonferroni‐corrected p=0.003; heterozygote vs. homozygote Bonferroni‐corrected p=0.032); Mir183 p=0.04 (wildtype vs. heterozygote Bonferroni‐corrected p=1.0; wildtype vs. homozygote Bonferroni‐corrected p=0.068; heterozygote vs. homozygote Bonferroni‐corrected p=0.094), Error bars are standard deviation (* = P < 0.05, ** = P ≤ 0.01). Supplementary Figure S2. Individual ABR thresholds of wildtype, heterozygous and homozygous Mir183/96dko mice at all ages tested. Number of mice of each genotype tested at each age is shown on the threshold plot. Supplementary Figure S3. Individual ABR thresholds of wildtype, heterozygous and homozygous Mir182ko mice at all ages tested.