5 XI November 2017
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

3768/SIC-MNR/2019 Date: - 16-11-2020
Telangana State Information Commission (Under Right to Information Act, 2005) D.No.5-4-399, Samachara Hakku Bhavan (Old ACB Building), Mojam-jahi-Market, Hyderabad – 500 001 Phone: 24740666 Fax: 24740592 Appeal No:3768/SIC-MNR/2019 Date: - 16-11-2020 Appellant : Sri U. Ekanth Goud, H.No. 3-3-18, Bagh Ameer, Kukatpally, Hyderabad - 500 072. Respondents : Public Information Officer (U/RTI Act, 2005) The Deputy Tahsildar, Bachupally Mandal, Medchal – Malkajgiri District. First Appellate Authority (U/RTI Act, 2005) The Tahsildar, Bachupally Mandal, Medchal – Malkajgiri District. Order Sri U. Ekanth Goud, H No. 3-3-18, Bagh Ameer, Kukatpally, Hyderabad- 500072 has filed 2nd appeal dated 27-03-2019 which was received by this Commission on 28-03-2019 for not getting the information sought by him from the PIO / The Deputy Tahsildar, Bachupally Mandal, Medchal – Malkajgiri District and 1st Appellate Authority / The Tahsildar, Bachupally Mandal, Medchal – Malkajgiri District. The brief facts of the case as per the appeal and other records received along with it are that the appellant herein filed an application dated 27-12-2018 before the PIO under Sec.6(1) of the RTI Act, 2005, requesting to furnish the information on the followingTSIC points mentioned in his application: The Public Information Officer / O/o. The Tahsildar, Mandal Revenue Office, Bachupally Mandal, Medchal Dist vide Lr.No.RTI/542/2019, dated 30-09-2019 has transferred the application u/s 6(3) of the RTI Act to the Public Information Officer / The Asst. Director, Survey and Land Records, Medchal Malkajgiri Dist to furnish the information directly to the Complainant under intimation to their office. -

230V Bus Time Schedule & Line Route
230V bus time schedule & line map 230V Vavilala Village to Secunderabad (Medchal View In Website Mode The 230V bus line (Vavilala Village to Secunderabad (Medchal) has 2 routes. For regular weekdays, their operation hours are: (1) Secunderabad (Medchal): 5:02 AM - 6:47 PM (2) Vavilala: 6:15 AM - 8:55 PM Use the Moovit App to ƒnd the closest 230V bus station near you and ƒnd out when is the next 230V bus arriving. Direction: Secunderabad (Medchal) 230V bus Time Schedule 25 stops Secunderabad (Medchal) Route Timetable: VIEW LINE SCHEDULE Sunday 5:02 AM - 6:47 PM Monday 5:02 AM - 6:47 PM Vavilala Tuesday 5:02 AM - 6:47 PM Khajapally Wednesday 5:02 AM - 6:47 PM Dundigal Thursday 5:02 AM - 6:47 PM Bahadurpally Friday 5:02 AM - 6:47 PM Katta Maisamma Saturday 5:02 AM - 6:47 PM Suraram X Road Saibad X Road 230V bus Info Jeedimetla Bus Depot Direction: Secunderabad (Medchal) Stops: 25 Trip Duration: 50 min Jeedimetla Substation Bus Stop Line Summary: Vavilala, Khajapally, Dundigal, Bahadurpally, Katta Maisamma, Suraram X Road, Shapur Nagar Saibad X Road, Jeedimetla Bus Depot, Jeedimetla Substation Bus Stop, Shapur Nagar, Hmt Factory, Hmt Factory Ganesh Nagar, Hal, Balanagar, Ferozeguda, New Bowenpally, Chinna Thokatta Request, Ashish Ganesh Nagar Gardens, Tar Bund Bus Stop, Pratap Colony, Caw O∆cers Mess Road, 2, Jubilee Bus Station, Patny, Hal Secunderabad Ymca, Secunderabad Bus Station (Gurudwara) Balanagar Ferozeguda New Bowenpally Chinna Thokatta Request NH44, Secunderabad Ashish Gardens NH44, Secunderabad Tar Bund Bus Stop Pratap Colony -

Greater Hyderabad Municipal Corporation Expression Of
GREATER HYDERABAD MUNICIPAL CORPORATION EXPRESSION OF INTEREST CUM REQUEST FOR PROPOSAL FOR “Empanelment of firm / agencies for construction of Sewage Treatment Plants (STPs) for Two BHK Housing Scheme of GHMC” NIT No: 05/SE-I(H)/GHMC/2018-19/ Dated: 10- 01-2019 Office Address: O/o. Superintending Engineer-I (Housing) Greater Hyderabad Municipal Corporation 3rd Floor, GHMC Building, Secunderabad: 500003 Email:[email protected] Ph No:9989930650,9177701934, 7995085328. Invitation for Proposal The Govt. in MA & UD Dept. have accorded Administrative sanction for taking up total 1,00,000 2BHK houses by GHMC in Hyderabad, Ranga Reddy, Medchal & Sanga Reddy District areas and GHMC has finalized 109 locations for taking up a total of 1,00,000 Houses in G+3, S+5 & C+S+9 Floors Pattern and entrusted to the different agencies by GHMC and the works are in progress. Greater Hyderabad Municipal Corporation (GHMC) desires for “Empanelment of firm / agencies for construction of Sewage Treatment Plants (STPs) for Two BHK Housing Scheme of GHMC” as per technical specifications and features mentioned in TABLE –A of this document at different locations in GHMC/HMDA area. Interested reputed Firms / Agencies, who have proven experience in construction of STPs and are able to provide O&M for 5 years are requested to participate in this Tender. About 50 STPs are to be constructed during the period from January-2019 to September-2019. The location of STPs proposed for the 2BHK Housing projects and the required capacity in MLD is shown in Annexure. The technical specifications, technical features of STP and other parameters are mentioned in TABLE-A of this document. -

India- Hyderabad- Residential Q4 2019
M A R K E T B E AT HYDERABAD Residential Q4 2019 New launches on the rise, more projects underway in 2020 A total of 4,340 new units were launched in Q4, a 2X rise on a quarterly basis. This is in line with our predictions during Q2-Q3 when several large- scale projects were awaiting approvals. Established catchments such as Kondapur, Hafeezpet, Nallagandla and fast-growing locations such as 14,464 NEW UNIT LAUNCHES (2019) Gopanpally – Tellapur, Bachupally are have witnessed new launches during the quarter. On an annual basis, the number of launches were higher by 30% y-o-y, suggesting a positive momentum in the residential market. Western quadrant accounted for 3/4th of the total launches during the year with majority of the projects launched within close proximity to IT and financial districts. Locations with major new launches in other parts of the SHARE OF MID SEGMENT IN NEW city include Uppal, Bolarum, Patancheru and Kismatpur etc. Mid segment projects accounted for nearly 90% of the units launched during the quarter 46% LAUNCHES (2019) in addition to a a luxury project in Kondapur. This sub-market continues to attract buyers in the premium segment on the back of its excellent physical and social infrastructure and proximity to IT and financial districts. Growth in new launches is likely to continue as developers are gearing up to launch nearly 20,000 units over the next 12-18 months. Several high-end projects with basic selling price exceeding INR 6000/sf were SHARE OF WESTERN QUADRANT IN launched in 2019, indicative of a demand shift and robust market fundamentals. -
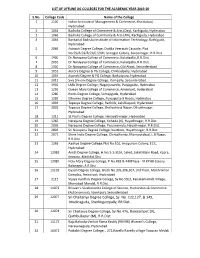
List of Offline UG Colleges for the Academic Year 2019-2020
LIST OF OFFLINE UG COLLEGES FOR THE ACADEMIC YEAR 2019-20 S.No. College Code Name of the College 1 1100 Indian Institute of Management & Commerce, Khairtabad, Hyderabad 2 1064 Badruka College of Commerce & Arts (Day), Kachiguda, Hyderabad 3 1065 Badruka College of Commerce & Arts (AN), Kachiguda, Hyderabad 4 1066 Bankatlal Badruka Institute of Information Technology, Kachiguda, Hyderabad 5 2086 Avinash Degree College, Dodda Veeraiah Cascade, Plot No.59/A,59/B,59/C,59/D, Srinagar Colony, Saroornagar, R.R.Dist 6 1400 Dr.Narayana College of Commerce, Kukatpally, R.R.Dist. 7 2091 Dr.Narayana College of Commerce, Kukatpally, R.R.Dist. 8 1300 Dr.Narayana College of Commerce, Old Alwal, Secunderabad 9 1051 Aurora Degree & PG College, Chikkadpally, Hyderabad 10 1059 Avanthi Degree & PG College, Barkatpura, Hyderabad 11 2012 Siva Shivani Degree College, Kompally, Secunderabad. 12 1901 LMA Degree College, Nagarjunahills, Panjagutta, Hyderabad 13 1293 Queen Mary College of Commerce, Ameerpet, Hyderabad 14 1280 Roots Degree College, Somajiguda, Hyderabad 15 1289 Ethames Degree College, Punjagutta X Roads, Hyderabad 16 1906 Tapasya Degree College, Redhills, Lakdikapool, Hyderabad 17 2802 Tapasya Degree College, Shalivahana Nagar, Dilsukhnagar, Hyderabad 18 1211 St.Paul’s Degree College, Himayathnagar, Hyderabad 19 1260 Narayana Degree College, Koheda (V), Hayathnagar, R.R.Dist. 20 2092 Narayana Degree College, Pasumamula, Hayathnagar, R.R.Dist. 21 2806 Sri Narayana Degree College, Kuntloor, Hayathnagar, R.R.Dist. 22 2071 Shine India Degree College, Chintalkunta, Mansoorabad, L.B Nagar, R.R.Dist. 23 1268 Aadhya Degree College,Plot No.101, Anupuram Colony, ECIL, Hyderabad 24 12081 Anish Degree College, H.No.1-1-31/A, Saket, Saket Main Road, Kapra, Keesara, Medchal Dist 25 12087 Holy Mary Degree College, P No.439 & 440Phase - VI KPHB Colony Balanagar, R R Dist. -

List of Applications to Be Placed in 46Th SEAC Meeting Scheduled on 26.09.2019 at O/O
List of Applications to be placed in 46th SEAC meeting scheduled on 26.09.2019 at O/o. TSPCB, Sanathnagar, Hyderabad. Date of S. No. Proposal No. Name of the Project Line of Activity Submission 1 04.02.2019 SIA/TG/NCP/94147/2019 "High Rised Residential Apartment" by P. Laxman Rao & Construction Project Others, Sy. No. 488, 489, & 490 (P), Kukatpally (V), Kukatpally (M), Medchal-Malkajgiri District. 2 29.04.2019 SIA/TG/MIS/103776/2019 "Infinity Commercial Complex" by M/s. Nayan Construction Project Construction, Sy. No. 90/2 (P), Gachibowli (V), Serilingampally (M), Rangareddy District. 3 26.04.2019 SIA/TG/MIS/103533/2019 Omsree Brilliance by M/s. Om Sree Builders & Developers, Sy. Construction Project No. 122, Yapral (V), Alwal (M), Medchal - Malkagiri District. 4 22.04.2019 SIA/TG/MIS/102958/2019 M/s. Urbanrise Builders LLP, Sy. No. 594/A, 595/A, 597/AA, Construction Project Dundigal (V), Gandimaisamma Dundigal (M), Medchal – Malkajgiri District. 5 16.04.2019 SIA/TG/MIS/102524/2019 M/s. Vensa Builders Private Limited, Sy. No. 319 (Part) & 320 Construction Project (Part), Puppalguda (V), Gandipet (M), Rangareddy District. 6 08.04.2019 SIA/TG/MIS/101676/2019 M/s. Mohd. Yakub and Others, Sy. No. 71 and 72, Hafeezpet Construction Project (V), Serilingampally (M), Rangareddy District. 7 04.04.2019 SIA/TG/MIS/101350/2019 M/s. Poulomi Estates Pvt. Ltd., Sy. No. 123, 125, 126 & 127, Construction Project Kokapet (V), Gandipet (M), Rangareddy District. 8 03.04.2019 SIA/TG/MIS/101239/2019 "Green Alfa" by M/s. -

Tentative Teachers List for Promotions - LB First Appointment Details Academic Qualifications Professional Qualifications
Personal Information Present Service Details DSC SNO Roster Employe Present School Present PHC Medium of Present School Date of Joining in Present Working No. (Treasury) Name of the Teacher Father's Name Gender Date of Birth Caste Designation Present working School Name Management Working (VH/HI/OH Post DISE code the present post District ID with subject (Govt/ LB) Mandal ) 1234 567 8 9 1011 12 13 14 15 16 17 ZPHS 1 1408709 U Kokilamba Veera Raghavaiah F 03-07-1964 OC SA Social Telugu ZPHS GandhiNagar 02-08-1985 LOCAL BODY Medchal NA Gandhinagar 2 1421191 S Vijay Kumar Padmaiah MALE 19-09-1966 BC-D L.F.L.H M TELUGU 36210900802 MPPS BHARAT NAGAR 19-12-1985 LOCAL BODY UPPAL MEDCHAL NO 3 1427190 K. PETER STEVEN MALE 09.06.1963 SC LFLHM TELUGU 36210990330 MPPS NAGOLE 28.07.1988 LOCAL BODY UPPAL Medchal-malkajigiri NILL 4 1431076 D.SUJANI D.NARSAIAH F 25-06-1966 BC-D S.A. SOCIAL TELUGU 36211100333 MPUPS KAMALA NAGAR 07-09-1989 LOCAL BODY KAPRA MEDCHAL - SEETHA RAMA 5 1431035 T.NANDINI F 20-11-1964 OC LFL HM TELUGU 36211100201 P.S CHINNA CHARLAPALLY 29-09-1989 LOCAL BODY KAPRA MEDCHAL SHARMA 6 144067 C.Ramchandraiah C. Ramaiah M 15.05.1965 SC LFL HM Telugu 36210300201 M.P.P.S.Nizampet 19.10.1989 LOCAL BODY Bachupally Medchal N.A 7 1438515 MALE VARIJA SUGNANI F 05-07-1963 BC-C LFL. H.M. TELUGU 36211100102 MPPS BALAJINAGAR 22-06-1990 LOCAL BODY KAPRA medchal - 8 1811982 KUTURU JAYASHRI LINGA REDDY F 06-05-1965 OC LFL. -

Details of Blos Appointed in Respect of Mahabub Nagar - Ranga Reddy - Hyderabad Graduates' Constituency
Details of BLOs appointed in respect of Mahabub Nagar - Ranga Reddy - Hyderabad Graduates' Constituency BLO Details Sl. Part Location of Building in which it will be District Name Polling Area No. No. Polling Station located Mobile Name of the BLO Designation Number 1 2 3 4 6 7 8 Zilla Parishad High School (S.Block) Village Revenue 1 Mahabubnagar 1 Koilkonda Entire Koilkonda Mandal B. Gopal 6303174951 Middle Room No.1 Assistant Zilla Parishad High School (S.Block) Village Revenue 2 Mahabubnagar 2 Koilkonda Entire Koilkonda Mandal B. Suresh 6303556670 Middle Room No.2 Assistant Govt., High School, Hanwada Ex Village Revenue 3 Mahabubnagar 3 Hanwada Hanwada Mandal J SHANKAR 9640619405 Mandal, Room No.2 Officer Govt., High School, Hanwada Ex Village Revenue 4 Mahabubnagar 4 Hanwada Hanwada Mandal K RAVINDAR 9182519739 Officer Mandal, Room No.3 Village Revenue 5 Mahabubnagar 5 Nawabpet ZPHS (Room No.1) Nawabpet Mandal S.RAJ KUMAR 9160331433 Assistant Village Revenue 6 Mahabubnagar 6 Nawabpet ZPHS (Room No.2) Nawabpet Mandal V SHEKAR 9000184469 Assistant Village Revenue 7 Mahabubnagar 7 Balanagar Mandal Primary School Balanagar Mandal B.Srisailam 9949053519 Assistant Village Revenue 8 Mahabubnagar 8 Rajapur ZPHS (Room No.1) Rajapur Mandal K.Ramu 9603656067 Assistant Ex Village Revenue 9 Mahabubnagar 9 Midjil ZPHS (Room No.2) Midjil Mandal SATYAM GOUD 9848952545 Officer Zilla Parishad High School Village Revenue 10 Mahabubnagar 10 Badepally Jadcherla Rural Villages SATHEESH 8886716611 (Boys), Room No.1 Assistant Zilla Parishad High School Village Revenue 11 Mahabubnagar 11 Badepally Jadcherla Rural Villages G SRINU 996303029 (Boys), Room No.2 Assistant Zilla Parishad High School Jadcherla Grama Village Revenue 12 Mahabubnagar 12 Badepally R.ANJANAMMA 9603804459 (Boys), Room No.3 Panchayath Paridhi Assistant 1 Details of BLOs appointed in respect of Mahabub Nagar - Ranga Reddy - Hyderabad Graduates' Constituency BLO Details Sl. -

List Police Station Under the District (Comma Separated) Printable District
Passport District Name DPHQ Name List of Pincode Under the District (Comma Separated) List Police Station Under the District (comma Separated) Printable District Saifabad, Ramgopalpet, Nampally, Abids , Begum Bazar , Narayanaguda, Chikkadpally, Musheerabad , Gandhi Nagar , Market, Marredpally, 500001, 500002, 500003, 500004, 500005, 500006, 500007, 500008, Trimulghery, Bollarum, Mahankali, Gopalapuram, Lallaguda, Chilkalguda, 500012, 500013, 500015, 500016, 500017, 500018, 500020, 500022, Bowenpally, Karkhana, Begumpet, Tukaramgate, Sulthan Bazar, 500023, 500024, 500025, 500026, 500027, 500028, 500029, 500030, Afzalgunj, Chaderghat, Malakpet, Saidabad, Amberpet, Kachiguda, 500031, 500033, 500034, 500035, 500036, 500038, 500039, 500040, Nallakunta, Osmania University, Golconda, Langarhouse, Asifnagar, Hyderabad Commissioner of Police, Hyderabad 500041, 500044, 500045, 500048, 500051, 500052, 500053, 500057, Hyderabad Tappachabutra, Habeebnagar, Kulsumpura, Mangalhat, Shahinayathgunj, 500058, 500059, 500060, 500061, 500062, 500063, 500064, 500065, Humayun Nagar, Panjagutta, Jubilee Hills, SR Nagar, Banjarahills, 500066, 500067, 500068, 500069, 500070, 500071, 500073, 500074, Charminar , Hussainialam, Kamatipura, Kalapather, Bahadurpura, 500076, 500077, 500079, 500080, 500082, 500085 ,500081, 500095, Chandrayangutta, Chatrinaka, Shalibanda, Falaknuma, Dabeerpura, 500011, 500096, 500009 Mirchowk, Reinbazar, Moghalpura, Santoshnagar, Madannapet , Bhavaninagar, Kanchanbagh 500005, 500008, 500018, 500019, 500030, 500032, 500033, 500046, Madhapur, -

Impact of Urban Growth on Water Bodies the Case of Hyderabad
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Research Papers in Economics Working Paper No. 60 September 2004 Impact of Urban Growth on Water Bodies The Case of Hyderabad C. Ramachandraiah Sheela Prasad CENTRE FOR ECONOMIC AND SOCIAL STUDIES Begumpet, Hyderabad-500016 1 Impact of Urban Growth on Water Bodies The Case of Hyderabad C. Ramachandraiah* Sheela Prasad** Abstract Being located in the Deccan Plateau region, Hyderabad city has been dotted with a number of lakes, which formed very important component of its physical environment. With the increasing control of the State and private agencies over the years, and rapid urban sprawl of the city, many of the water bodies have been totally lost. Many have been shrunk in size while the waters of several lakes got polluted with the discharge of untreated domestic and industrial effluents. This study makes an attempt to analyse the transformation of common property resources (the lakes) into private property. The adverse consequences of the loss of water bodies are felt in the steep decline in water table and the resultant water crisis in several areas. Further, the severity of flooding that was witnessed in August 2000 was also due to a reduction in the carrying capacity of lakes and water channels. The State has not bothered to either implement the existing laws or pay attention to the suggestions of environmental organisations in this regard. The paper argues that in this process of loss of water bodies in Hyderabad, the State is as much responsible as private agencies in terms of the policies that it has formulated and the lack of ensuring legislation and implementation. -

E-Auction Sale Notice Under Sarfaesi Act, 2002
Regional office, 2nd floor, opp: NIMS Hospital, Hyderabad _____________________ E-AUCTION SALE NOTICE UNDER SARFAESI ACT, 2002 Whereas the Bank acting through its Authorised Officer in exercise of its power under Section 13(4) of Securitisation and Reconstruction of Financial Assets and Enforcement of Security Interest Act, 2002 (SARFAESIA) decided to put up for sale, through online e-auction, the below mentioned scheduled properties for realisation of debts due to the Bank upon the following terms and conditions. The Authorised Officer has fixed the sale through online e- auction on 25.09.2018 through website https:// bankauctions.in provided by the service provider M/s.4 Closure, Hyderabad for recovery of an amount of Rs.1622377.00 (Rupees Sixteen lakhs twenty Two thousand Three hundred and seventy seven only) for Sl.No 1 and Rs.3277581.00 (Rupees Thirty Two Lakhs Seventy Seven Thousand Five Hundred and Eighty one only) {Rs. 2688861.00 As per Demand Notice DT. 08.02.2016} for Sl.No 2 and Rs.2296975.30 (Rupees Twenty Two Lakhs Ninety Six Thousand Nine Hundred and Seventy Five Paisa Thirty Only) {Rs. 2026116.30 as per Demand Notice Dt. 06.02.2017} for Sl.No 3 and Rs.2345913.08 (twenty three Lakhs Forty five Thousand Nine hundred and thirteen Paisa Eight Only) {Rs. 2155841.30 as per Demand Notice dt. 26.07.2017 for Sl.No 4 and Rs.2489528.80 (Rupees Twenty Four Lakhs Eighty Nine Thousand Five Hundred and twenty Eight Paisa Eighty Only) { Rs.2180583.00 As per Demand Notice DT. 01.06.2016} for Sl.No 5 and Rs.2007721.21 (Rupees Twenty Lakhs Seven Thousand Seven Hundred and Twenty One Paisa Twenty One Only) {Rs.1819423.40 As per Demand Notice DT. -

COVID-19 Dated: 21/08/2020 A
GOVERNMENTOF TELANGANA OFFICE OF THE DIRECTOR OF PUBLIC HEALTH AND FAMILY WELFARE MEDIA BULLETIN- COVID-19 Dated: 21/08/2020 As of:20/08/2020 (8PM) STATUS OF COVID-19 CASES S. NO DETAILS NUMBER 1. NO. OF POSITIVE CASES TODAY (CUMULATIVE) 1,967 (99,391) 2. NO. OF RECOVERED CASES TODAY (CUMULATIVE) 1,781 (76,967) 3. NO. OF DEATHS TODAY (CUMULATIVE) 8 (737) 4. CASE FATALITY RATE (INDIA) 0.74% (1.90%) 5. RECOVERY RATE (INDIA) 77.43 (73.91%) 6. TOTAL NUMBER OF ACTIVE CASES 21,687 7. NO. OF INDIVIDUALS IN HOME/INSTITUTIONAL ISOLATION 15,332 STATUS OF TESTS S. NO DETAILS NUMBER 1 NO. OF SAMPLES TESTED TODAY (CUMULATIVE) AGAINST DAILY TESTING 26,767 TARGET FOR TELANGANA AS PER W.H.O. BENCHMARK @ 140 PER MILLION PER DAY, I.E., 5,600 TESTS PER DAY (8,48,078) 2 SAMPLES TESTED PER MILLION POPULALATION 22,843 3 NO. OF REPORTS AWAITED 1,300 1 AGE AND GENDER WISE COVID POSITIVE DETAILS S.NO AGE GROUP AGE WISE POSITIVE CASES (%) TOTAL MALE FEMALE 1 UP TO 10 YEARS 3.4 2 1.4 2 11-20 YEARS 6.3 3.4 2.9 3 21-30 YEARS 22.9 14.8 8.1 4 31-40 YEARS 24.6 17.2 7.4 5 41-50 YEARS 18.4 12 6.4 6 51-60 YEARS 14.4 9.4 5 7 61-70 YEARS 7 4.3 2.7 8 71-80 YEARS 2.5 1.6 0.9 9 81 & ABOVE 0.5 0.4 0.1 TOTAL 100% 65.10% 34.90% COMORBIDITIES STATUS AMONG DEATHS PERCENTAGE OF DEATHS DUE TO COVID-19 46.13 % PERCENTAGE OF DEATHS DUE TO COMORBIDITIES 53.87 % DETAILS OF INFRASTRUCTURE/BED OCCUPANCY UNDER GOVERNMENT S.