“Clanwilliam Cedar” (Widdringtonia Cedarbergensis JA
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Contributions to the Life-History of Tetraclinis Articu- Lata, Masters, with Some Notes on the Phylogeny of the Cupressoideae and Callitroideae
Contributions to the Life-history of Tetraclinis articu- lata, Masters, with some Notes on the Phylogeny of the Cupressoideae and Callitroideae. BY W. T. SAXTON, M.A., F.L.S., Professor of Botany at the Ahmedabad Institute of Science, India. With Plates XLIV-XLVI and nine Figures in the Text. INTRODUCTION. HE Gum Sandarach tree of Morocco and Algeria has been well known T to botanists from very early times. Some account of it is given by Hooker and Ball (20), who speak of the beauty and durability of the wood, and state that they consider the tree to be probably correctly identified with the Bvlov of the Odyssey (v. 60),1 and with the Ovlov and Ovia of Theo- phrastus (' Hist. PI.' v. 3, 7)/ as well as, undoubtedly, with the Citrus wood of the Romans. The largest trees met with by them, growing in an un- cultivated state, were about 30 feet high. The resin, known as sandarach, is stated to be collected by the Moors and exported to Europe, where it is used as a varnish. They quote Shaw (49 a and b) as having described and figured the tree under the name of Thuja articulata, in his ' Travels in Barbary'; this statement, however, is not accurate. In both editions of the work cited the plant is figured and described as ' Cupressus fructu quadri- valvi, foliis Equiseti instar articulatis '. Some interesting particulars of the use of the timber are given by Hansen (19), who also implies that the embryo has from three to six cotyledons. Both Hooker and Ball, and Hansen, followed by almost all others who have studied the plant, speak of it as Callitris qtiadrivalvis. -

Botanic Gardens and Their Contribution to Sustainable Development Goal 15 - Life on Land Volume 15 • Number 2
Journal of Botanic Gardens Conservation International Volume 15 • Number 2 • July 2018 Botanic gardens and their contribution to Sustainable Development Goal 15 - Life on Land Volume 15 • Number 2 IN THIS ISSUE... EDITORS EDITORIAL: BOTANIC GARDENS AND SUSTAINABLE DEVELOPMENT GOAL 15 .... 02 FEATURES NEWS FROM BGCI .... 04 Suzanne Sharrock Paul Smith Director of Global Secretary General Programmes PLANT HUNTING TALES: SEED COLLECTING IN THE WESTERN CAPE OF SOUTH AFRICA .... 06 Cover Photo: Franklinia alatamaha is extinct in the wild but successfully grown in botanic gardens and arboreta FEATURED GARDEN: SOUTH AFRICA’S NATIONAL BOTANICAL GARDENS .... 09 (Arboretum Wespelaar) Design: Seascape www.seascapedesign.co.uk INTERVIEW: TALKING PLANTS .... 12 BGjournal is published by Botanic Gardens Conservation International (BGCI). It is published twice a year. Membership is open to all interested individuals, institutions and organisations that support the aims of BGCI. Further details available from: • Botanic Gardens Conservation International, Descanso ARTICLES House, 199 Kew Road, Richmond, Surrey TW9 3BW UK. Tel: +44 (0)20 8332 5953, Fax: +44 (0)20 8332 5956, E-mail: [email protected], www.bgci.org SUSTAINABLE DEVELOPMENT GOAL 15 • BGCI (US) Inc, The Huntington Library, Suzanne Sharrock .... 14 Art Collections and Botanical Gardens, 1151 Oxford Rd, San Marino, CA 91108, USA. Tel: +1 626-405-2100, E-mail: [email protected] SDG15: TARGET 15.1 Internet: www.bgci.org/usa AUROVILLE BOTANICAL GARDENS – CONSERVING TROPICAL DRY • BGCI (China), South China Botanical Garden, EVERGREEN FOREST IN INDIA 1190 Tian Yuan Road, Guangzhou, 510520, China. Paul Blanchflower .... 16 Tel: +86 20 85231992, Email: [email protected], Internet: www.bgci.org/china SDG 15: TARGET 15.3 • BGCI (Southeast Asia), Jean Linsky, BGCI Southeast Asia REVERSING LAND DEGRADATION AND DESERTIFICATION IN Botanic Gardens Network Coordinator, Dr. -

Cheŵa Women's Instructions in South-Central Africa
[Downloaded free from http://www.conservationandsociety.org on Monday, January 16, 2017, IP: 187.227.107.114] Conservation and Society 14(4): 406-415, 2016 Article Animals’ Role in Proper Behaviour: Cheŵa Women’s Instructions in South-Central Africa Leslie F. Zubieta Current affiliation: Centre for Rock Art Research + Management, The University of Western Australia, Perth, Western Australia, Australia E-mail: [email protected] Abstract The most common role of animals in the Cheŵa culture of south-central Africa is twofold: they are regarded as an important source of food, and they also provide raw materials for the creation of traditional medicines. Animals, however, also have a nuanced symbolic role that impacts the way people behave with each other by embodying cultural protocols of proper — and not so proper — behaviour. They appear repeatedly in storytelling and proverbs to reference qualities that people need to avoid or pursue and learn from the moral of the story in which animals interplay with each other, just as humans do. For example, someone who wants to prevent the consequences of greed is often advised to heed hyena stories and proverbs. My contribution elaborates on Brian Morris’s instrumental work in south-central Africa, which has permitted us to elucidate the symbolism of certain animals and the perception of landscape for Indigenous populations in this region. I discuss some of the ways in which animals have been employed to teach and learn proper behaviour in a particular sacred ceremony of the Cheŵa people which takes place in celebration of womanhood: Chinamwali. Keywords: Animal symbolism, initiation, Cheŵa, rock art, behaviour, Indigenous women, south-central Africa, Indigenous knowledge, Chinamwali INTRODUCTION highway. -

E-Cop18-Prop Draft-Widdringtonia
Original language: English CoP18 Doc. XXX CONVENTION ON INTERNATIONAL TRADE IN ENDANGERED SPECIES OF WILD FAUNA AND FLORA ____________________ Eighteenth meeting of the Conference of the Parties Colombo (Sri Lanka), 23 May – 3 June 2019 CONSIDERATION OF PROPOSALS FOR AMENDMENT OF APPENDICES II1 A. Proposal To list the species Widdringtonia whytei in CITES Appendix II without annotation specifying the types of specimens to be included, in order to include all readily recognizable parts and derivatives in accordance with Resolution Conf. 11.21 (Rev. CoP17). On the basis of available trade data and information it is known that the regulation of trade in the species is absolutely necessary to avoid this critically endangered species, with major replantation efforts underway, becoming eligible for inclusion in Appendix I in the very near future. B. Proponent Malawi C. Supporting Statement 1. Taxonomy 1.1. Class: Pinopsida 1.2. Order: Pinales 1.3. Family: Cupressaceae 1.4. Genus and species: Widdringtonia whytei Rendle 1.5. Scientific synonyms: Widdringtonia nodiflora variety whytei (Rendle) Silba 1.6. Common names: Mulanje cedar, Mulanje cedarwood, Mulanje cypress, Mkunguza 2. Overview Widdringtonia whytei or Mulanje cedar, the national tree of Malawi, is a conifer in the cypress family endemic to the upper altitude reaches of the Mount Mulanje massif (1500-2200 a.s.l.). This highly valued, decay- and termite-resistant species is considered to be “critically endangered” by the International Union for Conservation of Nature (IUCN) Red List of Threatened Species after years of overexploitation from unsustainable and illegal logging combined with human-induced changes to the fire regime, invasive competing tree species, aphid infestation, and low rates of 1 This document has been submitted by Malawi. -

Supporting Information
Supporting Information Mao et al. 10.1073/pnas.1114319109 SI Text BEAST Analyses. In addition to a BEAST analysis that used uniform Selection of Fossil Taxa and Their Phylogenetic Positions. The in- prior distributions for all calibrations (run 1; 144-taxon dataset, tegration of fossil calibrations is the most critical step in molecular calibrations as in Table S4), we performed eight additional dating (1, 2). We only used the fossil taxa with ovulate cones that analyses to explore factors affecting estimates of divergence could be assigned unambiguously to the extant groups (Table S4). time (Fig. S3). The exact phylogenetic position of fossils used to calibrate the First, to test the effect of calibration point P, which is close to molecular clocks was determined using the total-evidence analy- the root node and is the only functional hard maximum constraint ses (following refs. 3−5). Cordaixylon iowensis was not included in in BEAST runs using uniform priors, we carried out three runs the analyses because its assignment to the crown Acrogymno- with calibrations A through O (Table S4), and calibration P set to spermae already is supported by previous cladistic analyses (also [306.2, 351.7] (run 2), [306.2, 336.5] (run 3), and [306.2, 321.4] using the total-evidence approach) (6). Two data matrices were (run 4). The age estimates obtained in runs 2, 3, and 4 largely compiled. Matrix A comprised Ginkgo biloba, 12 living repre- overlapped with those from run 1 (Fig. S3). Second, we carried out two runs with different subsets of sentatives from each conifer family, and three fossils taxa related fi to Pinaceae and Araucariaceae (16 taxa in total; Fig. -

Widdringtonia Cedarbergensis)
The Conservation Genetics of the Clanwilliam Cedar (Widdringtonia cedarbergensis) by JANET THOMAS March 1995 Department of Botany Faculty of Science · UniversityUniversity ofof Cape Cape Town Town Submitted for the degree Master of Science c":·"="•''"<'':.-:.,,.c· . .". ''-~.c·c :.&.c.··'\:' ~;:·h£ Lt:i:.:~~·.:i:·~· ·.:·' r>:r·;· ~~.-~~~~- ::~· ... :..r;:c:n r.i~~~n t~·10 ~t~!~~ ~c ,.,_;,"\-~· ~: ..;·) t~:··; ~~<;:_::?; iii ,_..,.h0~e ~-~ or f:1 f·M:·t Ct~.->·::;;~:,: ;; :·; ... ~.~ L·~: 'Lh.:; r.:.:~ho~~ ·, The copyright of this thesis vests in the author. No quotation from it or information derived from it is to be published without full acknowledgement of the source. The thesis is to be used for private study or non- commercial research purposes only. Published by the University of Cape Town (UCT) in terms of the non-exclusive license granted to UCT by the author. University of Cape Town Acknowledgements I thank Professor William Bond, my supervisor, for guidance and inspiration throughout this project. Thanks are due to William Bond, Hans Nieuwmeyer, Doug Euston-Brown, Stan Ricketts, Camel, Maryke Honig and Lee Jones for their assistance in the field, particularly Doug and Stan for their death-defying rock climbs to precipitous sampling sites in the Baviaanskloof. I also thank Rob Scott-Shaw for providing seed from Cathedral Peak; Doug Jeffrey for showing me a couple of electrophoretic ropes; Robbie Robinson for his helpful comments on genetics; and Hans Nieuwmeyer for his engineering views on wind-pollination. This project was sponsored partly by FRD special programmes issued by The Percy Fitzpatrick Institute of Ornithology and mostly by South African Nature Foundation. -

Re-Examining the Past and Rethinking The
University of South Carolina Scholar Commons Theses and Dissertations 1-1-2013 Re-examining the Past and Rethinking the Future at Mount Mulanje Forest Reserve, Malawi: New Directions for Local Engagement Mary Christian Thompson University of South Carolina - Columbia Follow this and additional works at: https://scholarcommons.sc.edu/etd Part of the Educational Administration and Supervision Commons Recommended Citation Thompson, M. C.(2013). Re-examining the Past and Rethinking the Future at Mount Mulanje Forest Reserve, Malawi: New Directions for Local Engagement. (Doctoral dissertation). Retrieved from https://scholarcommons.sc.edu/etd/2497 This Open Access Dissertation is brought to you by Scholar Commons. It has been accepted for inclusion in Theses and Dissertations by an authorized administrator of Scholar Commons. For more information, please contact [email protected]. Re-examining the Past and Rethinking the Future at Mount Mulanje Forest Reserve, Malawi: New Directions for Local Engagement By Mary C. Thompson Bachelor of Arts University of Tennessee, 2004 Master of Arts University of South Carolina, 2008 Submitted in Partial Fulfillment of the Requirements For the Degree of Doctor of Philosophy in Geography College of Arts and Sciences University of South Carolina 2013 Accepted by: Edward R. Carr, Major Professor John Kupfer, Committee Member Brent McCusker, Committee Member Caroline Nagel, Committee Member Lacy Ford, Vice Provost and Dean of Graduate Studies © Copyright by Mary C. Thompson, 2013 All Rights Reserved. ii ACKNOWLEDGEMENTS I would like to acknowledge the immense support that the following people have given me during the preparation of this dissertation without which I would not have succeeded. -

Research Trials on Mulanje Cedar in Malawi
In forestry, it is well understood that Research trials on Mulanje different populations of trees within the natural range of a species can become adapted to local growing conditions. cedar in Malawi Comparing the survival and growth of trees raised from seed collected from by Dr Richard Jinks, Forest Research different populations can help select Malawi’s national tree is a conifer – the seed sources that are well adapted to the Mulanje cedar (Widdringtonia whytei). It conditions at a particular planting site. occurs naturally only on Mount Mulanje, The term ‘provenance’ is used in forestry which is a large granite massif in the south to refer to the particular place of origin east of Malawi that rises spectacularly of seeds of a species. At each trial site, above the plains and is the highest seedlings raised from three provenances mountain in southern Africa reaching over of Mulanje cedar are being tested to see 3000 m and covering about 650 km2. if any are better adapted than others to Mulanje cedar is critically endangered due particular soils and climates. Seedlings to a combination of threats including fire from the different provenances are and illegal logging for its valuable timber planted in a series of replicated small which is termite resistant and naturally plots (mini-plantations) that are set out in fragrant (Figure 1). a random arrangement across each site (Figure 4). The randomisation procedure reduces accidental bias in the results caused by variation in soil fertility or other site factors. Figure 3. Locations (red) of the trial sites planted in 2017. -
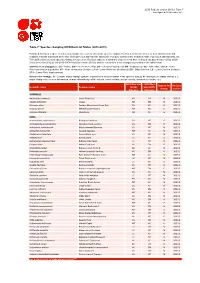
Table 7: Species Changing IUCN Red List Status (2012-2013)
IUCN Red List version 2013.2: Table 7 Last Updated: 25 November 2013 Table 7: Species changing IUCN Red List Status (2012-2013) Published listings of a species' status may change for a variety of reasons (genuine improvement or deterioration in status; new information being available that was not known at the time of the previous assessment; taxonomic changes; corrections to mistakes made in previous assessments, etc. To help Red List users interpret the changes between the Red List updates, a summary of species that have changed category between 2012 (IUCN Red List version 2012.2) and 2013 (IUCN Red List version 2013.2) and the reasons for these changes is provided in the table below. IUCN Red List Categories: EX - Extinct, EW - Extinct in the Wild, CR - Critically Endangered, EN - Endangered, VU - Vulnerable, LR/cd - Lower Risk/conservation dependent, NT - Near Threatened (includes LR/nt - Lower Risk/near threatened), DD - Data Deficient, LC - Least Concern (includes LR/lc - Lower Risk, least concern). Reasons for change: G - Genuine status change (genuine improvement or deterioration in the species' status); N - Non-genuine status change (i.e., status changes due to new information, improved knowledge of the criteria, incorrect data used previously, taxonomic revision, etc.) IUCN Red List IUCN Red Reason for Red List Scientific name Common name (2012) List (2013) change version Category Category MAMMALS Nycticebus javanicus Javan Slow Loris EN CR N 2013.2 Okapia johnstoni Okapi NT EN N 2013.2 Pteropus niger Greater Mascarene Flying -

Tembo Chanyenga Phd Thesis Final for SU Library 20130902
EFFECT OF POPULATION SIZE ON VIABLE SEED OUTPUT, SEED RAIN AND NATURAL REGENERATION PATTERN OF A TROPICAL CONIFER WIDDRINGTONIA WHYTEI - RENDLE IN MALAWI by Tembo Faera Chanyenga Dissertation presented for the degree of Doctor of Philosophy (Forest Science) in Department of Forest and Wood Science Faculty of AgriSciences Stellenbosch University, Stellenbosch, South Africa Supervisor: Prof. Coert Johannes Geldenhuys Co-Supervisor: Dr. Moctar Sacande DECEMBER 2013 Stellenbosch University http://scholar.sun.ac.za DECLARATION By submitting this dissertation electronically, I declare that the entirety of the work contained therein is my own, original work, that I am the sole author thereof (save to the extent explicitly otherwise stated), that reproduction and publication thereof by Stellenbosch University will not infringe any third party rights and that I have not previously in its entirety or in part submitted it for obtaining any qualification. 4 September 2013 Copyright © 2013 Stellenbosch University All rights reserved i Stellenbosch University http://scholar.sun.ac.za ABSTRACT Widdringtonia whytei is a tropical endemic, fire-adapted pioneer coniferous tree species within natural fire-fragmented Afromontane forest patches in a confined area on Mulanje Mountain in Malawi. Natural and anthropogenic fires within the surrounding fire-prone landscape, insect attacks, and uncontrolled harvesting of mature trees for timber threaten the survival of W. whytei. This study investigated the effects of population fragmentation on the reproductive biology of W. whytei, through four specific studies: effects of population size, tree size and crown position on viable seed output; seed rain variation among population sizes; effects of temperature and light on viability and germination of W. -

Highlights from the IUCN Red List of Threatened Species 2013.1 Update
Highlights from the IUCN Red List of Threatened Species 2013.1 update Species moving to Critically Endangered (Possibly Extinct) • Crustaceans o Florida Cave Shrimp Palaemonetes cummingi – moved from Vulnerable to Critically Endangered (Possibly Extinct) • Plants o Podocarpus perrieri– moved from Critically Endangered to Critically Endangered (Possibly Extinct) Re-discovered species o Fleischmann’s Robber Frog Craugastor fleischmanni – moved from Critically Endangered (Possibly Extinct) to Critically Endangered o Starrett’s Treefrog Isthmohyla tica - moved from Critically Endangered (Possibly Extinct) to Critically Endangered Status changes • Amphibians o Bale Mountains Treefrog Balebreviceps hillmani – moved from Endangered to Critically Endangered • Freshwater Fish o Yaqui Catfish Ictalurus pricei – moved from Vulnerable to Endangered o Conasauga Loach Percina jenkinsi – moved from Vulnerable to Critically Endangered o Woundfin Plagopterus argentissimus – moved from Vulnerable to Critically Endangered • Plants o Whitebark Pine Pinus albicaulis – moved from Vulnerable to Endangered o Blue Mountain Yacca Podocarpus urbanii– moved from Near Threatened to Critically Endangered o Mulanje Cedar Widdringtonia whytei – moved from Endangered to Critically Endangered Some examples of the over 4,800 species newly recorded on the 2013.1 IUCN Red List • Amphibians o Overlooked Squeaker Frog Arthroleptis kutogundua – enters as Critically Endangered (Possibly Extinct) • Invertebrates o Golden Sandfish Holothuria lessoni – enters as Endangered o Kanthan Cave Trapdoor Spider Liphistius kanthan – enters as Critically Endangered • Reptiles o Vitilevu Mountain Treeskink Emoia campbelii – enters as Endangered o Ono-i-Lau Ground Skink Leiolopisma alazon – enters as Critically Endangered o Helmethead Gecko Tarentola chazaliae – enters as Vulnerable • Plants o Hill Turmeric Curcuma pseudomontana – enters as Vulnerable o Linum katiae – enters as Vulnerable o Masdevallia atahualpa – enters as Endangered o Nepenthes suratensis – enters as Critically Endangered . -

The Plight of the Mount Mulanje Cedar Widdringtonia Whytei in Malawi
Oryx Vol 41 No 1 January 2007 Saving the Island in the Sky: the plight of the Mount Mulanje cedar Widdringtonia whytei in Malawi Julian Bayliss, Steve Makungwa, Joy Hecht, David Nangoma and Carl Bruessow Abstract The Endangered Mulanje cedar Widdringtonia which represents a loss of 616.7 ha over the previous whytei, endemic to the Mount Mulanje massif in Malawi, 15 years. Of the recorded trees 32.27% (37,242 m3) were has undergone a drastic decline due to increased fire dead cedars. Therefore, under current Department of incidence and illegal logging. Valued for its fine timber, Forestry harvest licensing, there remains in theory attractive fragrance, and pesticide-resistant sap, the tree sufficient dead cedar to last .30 years. At this stage it has been regarded as highly desirable since its discovery is imperative that cedar nurseries are established and in the late 19th century. Because of its steep slopes and saplings planted out across the mountain on an annual isolated high altitude plateau, Mount Mulanje is also a basis, small cedar clusters are protected to facilitate refuge for a number of other endemic plant species. The regeneration, and a strict monitoring programme is first assessment of the Mulanje cedar since 1994 was followed to prevent the cutting of live cedar. commissioned by the Mulanje Mountain Conservation Trust to ascertain the species’ current extent and status. Keywords Fire, Malawi, Mount Mulanje, Mulanje This study identified an area of 845.3 ha of Mulanje cedar, cedar, Widdringtonia whytei. Introduction late President Dr Hastings Banda, and has come to symbolize the delicate balance that faces the Mount The Mulanje cedar was first noted by the Scottish Mulanje ecosystem.