Comparative Analysis of Two C-Terminal Kinesin Motor Proteins: KIFC1 and KIFC5A
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Kinesin-14 Motor Protein KIFC1 Participates in DNA Synthesis and Chromatin Maintenance Ya-Lan Wei1 and Wan-Xi Yang 1
Wei and Yang Cell Death and Disease (2019) 10:402 https://doi.org/10.1038/s41419-019-1619-9 Cell Death & Disease ARTICLE Open Access Kinesin-14 motor protein KIFC1 participates in DNA synthesis and chromatin maintenance Ya-Lan Wei1 and Wan-Xi Yang 1 Abstract The nuclear localization signal (NLS) in kinesin-14 KIFC1 is associated with nuclear importins and Ran gradient, but detailed mechanism remains unknown. In this study, we found that KIFC1 proteins have specific transport characteristics during cell cycle. In the absence of KIFC1, cell cycle kinetics decrease significantly with a prolonged S phase. After KIFC1 overexpression, the duration of S phase becomes shorten. KIFC1 may transport the recombinant/ replicate-related proteins into the nucleus, meanwhile avoiding excessive KIFC1 in the cytoplasm, which results in aberrant microtubule bundling. Interestingly, the deletion of kifc1 in human cells results in a higher ratio of aberrant nuclear membrane, and the degradation of lamin B and lamin A/C. We also found that kifc1 deletion leads to defects in metaphase mitotic spindle assembly, and then results in chromosome structural abnormality. The kifc1-/- cells finally form micronuclei in daughter cells, and results in aneuploidy and chromosome loss in cell cycle. In this study, we demonstrate that kinesin-14 KIFC1 proteins involve in regulating DNA synthesis in S phase, and chromatin maintenance in mitosis, and maintain cell growth in a nuclear transport-independent way. 1234567890():,; 1234567890():,; 1234567890():,; 1234567890():,; Introduction KIFC1 mainly cluster the spindles involving in chromo- Kinesin-14 KIFC1 transports various cargos along the some alignment and segregation. While chromokinesins microtubule to the minus ends1. -

Viewed Under 23 (B) Or 203 (C) fi M M Male Cko Mice, and Largely Unaffected Magni Cation; Scale Bars, 500 M (B) and 50 M (C)
BRIEF COMMUNICATION www.jasn.org Renal Fanconi Syndrome and Hypophosphatemic Rickets in the Absence of Xenotropic and Polytropic Retroviral Receptor in the Nephron Camille Ansermet,* Matthias B. Moor,* Gabriel Centeno,* Muriel Auberson,* † † ‡ Dorothy Zhang Hu, Roland Baron, Svetlana Nikolaeva,* Barbara Haenzi,* | Natalya Katanaeva,* Ivan Gautschi,* Vladimir Katanaev,*§ Samuel Rotman, Robert Koesters,¶ †† Laurent Schild,* Sylvain Pradervand,** Olivier Bonny,* and Dmitri Firsov* BRIEF COMMUNICATION *Department of Pharmacology and Toxicology and **Genomic Technologies Facility, University of Lausanne, Lausanne, Switzerland; †Department of Oral Medicine, Infection, and Immunity, Harvard School of Dental Medicine, Boston, Massachusetts; ‡Institute of Evolutionary Physiology and Biochemistry, St. Petersburg, Russia; §School of Biomedicine, Far Eastern Federal University, Vladivostok, Russia; |Services of Pathology and ††Nephrology, Department of Medicine, University Hospital of Lausanne, Lausanne, Switzerland; and ¶Université Pierre et Marie Curie, Paris, France ABSTRACT Tight control of extracellular and intracellular inorganic phosphate (Pi) levels is crit- leaves.4 Most recently, Legati et al. have ical to most biochemical and physiologic processes. Urinary Pi is freely filtered at the shown an association between genetic kidney glomerulus and is reabsorbed in the renal tubule by the action of the apical polymorphisms in Xpr1 and primary fa- sodium-dependent phosphate transporters, NaPi-IIa/NaPi-IIc/Pit2. However, the milial brain calcification disorder.5 How- molecular identity of the protein(s) participating in the basolateral Pi efflux remains ever, the role of XPR1 in the maintenance unknown. Evidence has suggested that xenotropic and polytropic retroviral recep- of Pi homeostasis remains unknown. Here, tor 1 (XPR1) might be involved in this process. Here, we show that conditional in- we addressed this issue in mice deficient for activation of Xpr1 in the renal tubule in mice resulted in impaired renal Pi Xpr1 in the nephron. -

Kinesin Family Member 18B Regulates the Proliferation and Invasion Of
Wu et al. Cell Death and Disease (2021) 12:302 https://doi.org/10.1038/s41419-021-03582-2 Cell Death & Disease ARTICLE Open Access Kinesin family member 18B regulates the proliferation and invasion of human prostate cancer cells Yu-Peng Wu 1,Zhi-BinKe 1, Wen-Cai Zheng 1, Ye-Hui Chen 1,Jun-MingZhu 1,FeiLin 1,Xiao-DongLi 1, Shao-Hao Chen 1,HaiCai 1, Qing-Shui Zheng 1, Yong Wei 1, Xue-Yi Xue 1 and Ning Xu 1 Abstract Expression of kinesin family member 18B (KIF18B), an ATPase with key roles in cell division, is deregulated in many cancers, but its involvement in prostate cancer (PCa) is unclear. Here, we investigated the expression and function of KIF18B in human PCa specimens and cell lines using bioinformatics analyses, immunohistochemical and immunofluorescence microscopy, and RT-qPCR and western blot analyses. KIF18B was overexpressed in PCa specimens compared with paracancerous tissues and was associated with poorer disease-free survival. In vitro, KIF18B knockdown in PCa cell lines promoted cell proliferation, migration, and invasion, and inhibited cell apoptosis, while KIF18B overexpression had the opposite effects. In a mouse xenograft model, KIF18B overexpression accelerated and promoted the growth of PCa tumors. Bioinformatics analysis of control and KIF18B-overexpressing PCa cells showed that genes involved in the PI3K–AKT–mTOR signaling pathway were significantly enriched among the differentially expressed genes. Consistent with this observation, we found that KIF18B overexpression activates the PI3K–AKT–mTOR signaling pathway in PCa cells both in vitro and in vivo. Collectively, our results suggest that KIF18B plays a crucial role – – 1234567890():,; 1234567890():,; 1234567890():,; 1234567890():,; in PCa via activation of the PI3K AKT mTOR signaling pathway, and raise the possibility that KIF18B could have utility as a novel biomarker for PCa. -

Table 2. Significant
Table 2. Significant (Q < 0.05 and |d | > 0.5) transcripts from the meta-analysis Gene Chr Mb Gene Name Affy ProbeSet cDNA_IDs d HAP/LAP d HAP/LAP d d IS Average d Ztest P values Q-value Symbol ID (study #5) 1 2 STS B2m 2 122 beta-2 microglobulin 1452428_a_at AI848245 1.75334941 4 3.2 4 3.2316485 1.07398E-09 5.69E-08 Man2b1 8 84.4 mannosidase 2, alpha B1 1416340_a_at H4049B01 3.75722111 3.87309653 2.1 1.6 2.84852656 5.32443E-07 1.58E-05 1110032A03Rik 9 50.9 RIKEN cDNA 1110032A03 gene 1417211_a_at H4035E05 4 1.66015788 4 1.7 2.82772795 2.94266E-05 0.000527 NA 9 48.5 --- 1456111_at 3.43701477 1.85785922 4 2 2.8237185 9.97969E-08 3.48E-06 Scn4b 9 45.3 Sodium channel, type IV, beta 1434008_at AI844796 3.79536664 1.63774235 3.3 2.3 2.75319499 1.48057E-08 6.21E-07 polypeptide Gadd45gip1 8 84.1 RIKEN cDNA 2310040G17 gene 1417619_at 4 3.38875643 1.4 2 2.69163229 8.84279E-06 0.0001904 BC056474 15 12.1 Mus musculus cDNA clone 1424117_at H3030A06 3.95752801 2.42838452 1.9 2.2 2.62132809 1.3344E-08 5.66E-07 MGC:67360 IMAGE:6823629, complete cds NA 4 153 guanine nucleotide binding protein, 1454696_at -3.46081884 -4 -1.3 -1.6 -2.6026947 8.58458E-05 0.0012617 beta 1 Gnb1 4 153 guanine nucleotide binding protein, 1417432_a_at H3094D02 -3.13334396 -4 -1.6 -1.7 -2.5946297 1.04542E-05 0.0002202 beta 1 Gadd45gip1 8 84.1 RAD23a homolog (S. -

A Computational Approach for Defining a Signature of Β-Cell Golgi Stress in Diabetes Mellitus
Page 1 of 781 Diabetes A Computational Approach for Defining a Signature of β-Cell Golgi Stress in Diabetes Mellitus Robert N. Bone1,6,7, Olufunmilola Oyebamiji2, Sayali Talware2, Sharmila Selvaraj2, Preethi Krishnan3,6, Farooq Syed1,6,7, Huanmei Wu2, Carmella Evans-Molina 1,3,4,5,6,7,8* Departments of 1Pediatrics, 3Medicine, 4Anatomy, Cell Biology & Physiology, 5Biochemistry & Molecular Biology, the 6Center for Diabetes & Metabolic Diseases, and the 7Herman B. Wells Center for Pediatric Research, Indiana University School of Medicine, Indianapolis, IN 46202; 2Department of BioHealth Informatics, Indiana University-Purdue University Indianapolis, Indianapolis, IN, 46202; 8Roudebush VA Medical Center, Indianapolis, IN 46202. *Corresponding Author(s): Carmella Evans-Molina, MD, PhD ([email protected]) Indiana University School of Medicine, 635 Barnhill Drive, MS 2031A, Indianapolis, IN 46202, Telephone: (317) 274-4145, Fax (317) 274-4107 Running Title: Golgi Stress Response in Diabetes Word Count: 4358 Number of Figures: 6 Keywords: Golgi apparatus stress, Islets, β cell, Type 1 diabetes, Type 2 diabetes 1 Diabetes Publish Ahead of Print, published online August 20, 2020 Diabetes Page 2 of 781 ABSTRACT The Golgi apparatus (GA) is an important site of insulin processing and granule maturation, but whether GA organelle dysfunction and GA stress are present in the diabetic β-cell has not been tested. We utilized an informatics-based approach to develop a transcriptional signature of β-cell GA stress using existing RNA sequencing and microarray datasets generated using human islets from donors with diabetes and islets where type 1(T1D) and type 2 diabetes (T2D) had been modeled ex vivo. To narrow our results to GA-specific genes, we applied a filter set of 1,030 genes accepted as GA associated. -

Dynamics of the Linc Complex
DYNAMICS OF THE LINC COMPLEX Loic Gazquez University of Manchester School of Biological Sciences 2017 A thesis submitted to the University of Manchester for the degree of Doctor of Philosophy in the Faculty of Biology, Medicine and Health TABLE OF CONTENTS Table of Contents .................................................................................................................................... 2 Abstract.................................................................................................................................................... 4 Declaration .............................................................................................................................................. 5 Copyright statement ................................................................................................................................ 5 Acknowledgements ................................................................................................................................. 6 I. Introduction .................................................................................................................................. 7 Nuclear Envelope and the LINC complex ................................................................................... 7 I. 1.1. The first LINC component: the SUN ................................................................................ 8 I. 1.2. The second LINC component: the KASH ........................................................................ 8 I. 1.1. Structure -

XP725 Antibody List Lot 129K4830
Panorama® Antibody Microarray-XPRESS Profiler725, XP725 List of Antibodies Lot: 129K4830 Reactivity Number Antibody Sigma No. Gene Ids Gene Symbols P/M Accessions Human Mouse Rat 3 Actin A5060 58 ACTA1 P NP_001091.1 n/d n/d n/d 5 Actin, αβ-Smooth Muscle A5228 59 ACTA2 M NP_001135417.1, NP_001604.1, yyy 6 β-Actin A1978 60 ACTB M NP_001092.1 yyy 7 β-Actin A2228 60 ACTB M NP_001092.1 yyy 8 a-Actinin A5044 87 ACTN1 M NP_001093.1 y y n/d 555 PARP P7605 142 PARP1 P NP_001609.2 y n/d n/d 11 β1 and β2-Adaptins A4450 162 AP1B1 M NP_001118.3 y n/d y 167 Chondroitin Sulfate C8035 176 ACAN M NP_001126.3, NP_037359.3 yyy 574 Protein Kinase Ba /Akt1 P2482 207 AKT1 M NP_001014431.1,NP_001014432.1 , NP_005154.2, yyy 575 Protein Kinase Ba /Akt1 P1601 207 AKT1 P NP_001014431.1,NP_001014432.1 , NP_005154.2, yyy 577 phospho-PKB (pSer473) P4112 207 AKT1 P NP_001014431.1,NP_001014432.1 , NP_005154.2, n/d y y 578 phospho-PKB (pThr308) P3862 207 AKT1 P NP_001014431.1,NP_001014432.1 , NP_005154.2, n/d y y 22 Annexin V A8604 308 ANXA5 M NP_001145.1 y n/d n/d 23 Annexin VII A4475 310 ANXA7 M NP_001147.1 yyy 29 Apaf1, N-Terminal A8469 317 APAF1 P NP_001151.1 y y n/d 457 Mint2 M3319 321 APBA2 P NP_001123886.1 n/d n/d y 28 AP Endonuclease A2105 328 APEX1 M NP_001632.2,NP_542379.1,NP_542380.1 yyy 692 Survivin S8191 332 BIRC5 P NP_001012270.1 yyy 17 β-Amyloid A8354 351 APP M NP_000475.1,NP_001129488.1 ,NP_001129601.1 ,NP_001129602.1 ,NP_001129603.1 ,NP_001191230.1 ,NP_001191231.1 ,NP_001191232.1,NP_958816.1,NP_9 y n/d n/d 18 Amyloid Precursor Protein, C-Terminal A8717 -

Cellular and Molecular Signatures in the Disease Tissue of Early
Cellular and Molecular Signatures in the Disease Tissue of Early Rheumatoid Arthritis Stratify Clinical Response to csDMARD-Therapy and Predict Radiographic Progression Frances Humby1,* Myles Lewis1,* Nandhini Ramamoorthi2, Jason Hackney3, Michael Barnes1, Michele Bombardieri1, Francesca Setiadi2, Stephen Kelly1, Fabiola Bene1, Maria di Cicco1, Sudeh Riahi1, Vidalba Rocher-Ros1, Nora Ng1, Ilias Lazorou1, Rebecca E. Hands1, Desiree van der Heijde4, Robert Landewé5, Annette van der Helm-van Mil4, Alberto Cauli6, Iain B. McInnes7, Christopher D. Buckley8, Ernest Choy9, Peter Taylor10, Michael J. Townsend2 & Costantino Pitzalis1 1Centre for Experimental Medicine and Rheumatology, William Harvey Research Institute, Barts and The London School of Medicine and Dentistry, Queen Mary University of London, Charterhouse Square, London EC1M 6BQ, UK. Departments of 2Biomarker Discovery OMNI, 3Bioinformatics and Computational Biology, Genentech Research and Early Development, South San Francisco, California 94080 USA 4Department of Rheumatology, Leiden University Medical Center, The Netherlands 5Department of Clinical Immunology & Rheumatology, Amsterdam Rheumatology & Immunology Center, Amsterdam, The Netherlands 6Rheumatology Unit, Department of Medical Sciences, Policlinico of the University of Cagliari, Cagliari, Italy 7Institute of Infection, Immunity and Inflammation, University of Glasgow, Glasgow G12 8TA, UK 8Rheumatology Research Group, Institute of Inflammation and Ageing (IIA), University of Birmingham, Birmingham B15 2WB, UK 9Institute of -

RET Gene Fusions in Malignancies of the Thyroid and Other Tissues
G C A T T A C G G C A T genes Review RET Gene Fusions in Malignancies of the Thyroid and Other Tissues Massimo Santoro 1,*, Marialuisa Moccia 1, Giorgia Federico 1 and Francesca Carlomagno 1,2 1 Department of Molecular Medicine and Medical Biotechnology, University of Naples “Federico II”, 80131 Naples, Italy; [email protected] (M.M.); [email protected] (G.F.); [email protected] (F.C.) 2 Institute of Endocrinology and Experimental Oncology of the CNR, 80131 Naples, Italy * Correspondence: [email protected] Received: 10 March 2020; Accepted: 12 April 2020; Published: 15 April 2020 Abstract: Following the identification of the BCR-ABL1 (Breakpoint Cluster Region-ABelson murine Leukemia) fusion in chronic myelogenous leukemia, gene fusions generating chimeric oncoproteins have been recognized as common genomic structural variations in human malignancies. This is, in particular, a frequent mechanism in the oncogenic conversion of protein kinases. Gene fusion was the first mechanism identified for the oncogenic activation of the receptor tyrosine kinase RET (REarranged during Transfection), initially discovered in papillary thyroid carcinoma (PTC). More recently, the advent of highly sensitive massive parallel (next generation sequencing, NGS) sequencing of tumor DNA or cell-free (cfDNA) circulating tumor DNA, allowed for the detection of RET fusions in many other solid and hematopoietic malignancies. This review summarizes the role of RET fusions in the pathogenesis of human cancer. Keywords: kinase; tyrosine kinase inhibitor; targeted therapy; thyroid cancer 1. The RET Receptor RET (REarranged during Transfection) was initially isolated as a rearranged oncoprotein upon the transfection of a human lymphoma DNA [1]. -

Supplementary Table S4. FGA Co-Expressed Gene List in LUAD
Supplementary Table S4. FGA co-expressed gene list in LUAD tumors Symbol R Locus Description FGG 0.919 4q28 fibrinogen gamma chain FGL1 0.635 8p22 fibrinogen-like 1 SLC7A2 0.536 8p22 solute carrier family 7 (cationic amino acid transporter, y+ system), member 2 DUSP4 0.521 8p12-p11 dual specificity phosphatase 4 HAL 0.51 12q22-q24.1histidine ammonia-lyase PDE4D 0.499 5q12 phosphodiesterase 4D, cAMP-specific FURIN 0.497 15q26.1 furin (paired basic amino acid cleaving enzyme) CPS1 0.49 2q35 carbamoyl-phosphate synthase 1, mitochondrial TESC 0.478 12q24.22 tescalcin INHA 0.465 2q35 inhibin, alpha S100P 0.461 4p16 S100 calcium binding protein P VPS37A 0.447 8p22 vacuolar protein sorting 37 homolog A (S. cerevisiae) SLC16A14 0.447 2q36.3 solute carrier family 16, member 14 PPARGC1A 0.443 4p15.1 peroxisome proliferator-activated receptor gamma, coactivator 1 alpha SIK1 0.435 21q22.3 salt-inducible kinase 1 IRS2 0.434 13q34 insulin receptor substrate 2 RND1 0.433 12q12 Rho family GTPase 1 HGD 0.433 3q13.33 homogentisate 1,2-dioxygenase PTP4A1 0.432 6q12 protein tyrosine phosphatase type IVA, member 1 C8orf4 0.428 8p11.2 chromosome 8 open reading frame 4 DDC 0.427 7p12.2 dopa decarboxylase (aromatic L-amino acid decarboxylase) TACC2 0.427 10q26 transforming, acidic coiled-coil containing protein 2 MUC13 0.422 3q21.2 mucin 13, cell surface associated C5 0.412 9q33-q34 complement component 5 NR4A2 0.412 2q22-q23 nuclear receptor subfamily 4, group A, member 2 EYS 0.411 6q12 eyes shut homolog (Drosophila) GPX2 0.406 14q24.1 glutathione peroxidase -

Nuclear Envelope Laminopathies: Evidence for Developmentally Inappropriate Nuclear Envelope-Chromatin Associations
Nuclear Envelope Laminopathies: Evidence for Developmentally Inappropriate Nuclear Envelope-Chromatin Associations by Jelena Perovanovic M.S. in Molecular Biology and Physiology, September 2009, University of Belgrade M.Phil. in Molecular Medicine, August 2013, The George Washington University A Dissertation submitted to The Faculty of The Columbian College of Arts and Sciences of The George Washington University in partial fulfillment of the requirements for the degree of Doctor of Philosophy August 31, 2015 Dissertation directed by Eric P. Hoffman Professor of Integrative Systems Biology The Columbian College of Arts and Sciences of The George Washington University certifies that Jelena Perovanovic has passed the Final Examination for the degree of Doctor of Philosophy as of May 5, 2015. This is the final and approved form of the dissertation. Nuclear Envelope Laminopathies: Evidence for Developmentally Inappropriate Nuclear Envelope-Chromatin Associations Jelena Perovanovic Dissertation Research Committee: Eric P. Hoffman, Professor of Integrative Systems Biology, Dissertation Director Anamaris Colberg-Poley, Professor of Integrative Systems Biology, Committee Member Robert J. Freishtat, Associate Professor of Pediatrics, Committee Member Vittorio Sartorelli, Senior Investigator, National Institutes of Health, Committee Member ii © Copyright 2015 by Jelena Perovanovic All rights reserved iii Acknowledgments I am deeply indebted to countless individuals for their support and encouragement during the past five years of graduate studies. First and foremost, I would like to express my gratitude to my mentor, Dr. Eric P. Hoffman, for his unwavering support and guidance, and keen attention to my professional development. This Dissertation would not have been possible without the critical input he provided and the engaging environment he created. -
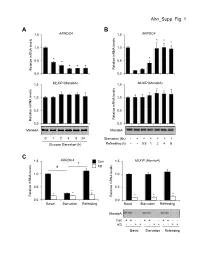
Ahn Supp. Fig. 1 AB 1.5 ARRDC4 1.5 ARRDC4 * * * 1.0 1.0
Ahn_Supp. Fig. 1 AB 1.5 ARRDC4 1.5 ARRDC4 * * * 1.0 1.0 * * 0.5 * 0.5 * * * Relative mRNA levels mRNA Relative Relative mRNA levels mRNA Relative 0.0 0.0 1.5 MLXIP (MondoA) 1.5 MLXIP (MondoA) 1.0 1.0 0.5 0.5 Relative mRNA levels mRNA Relative Relative mRNA levels mRNA Relative 0.0 0.0 MondoA MondoA 0124824 Starvation (6h) -++++++ Glucose Starvation (h) Refeeding (h) --0.51248 C 1.5 ARRDC4 1.5 MLXIP (MondoA) † Con # KD 1.0 1.0 0.5 0.5 * * * * Relative mRNA levels mRNA Relative Relative mRNA levels mRNA Relative * * 0.0 0.0 BasalStarvation Refeeding BasalStarvation Refeeding MondoA Con + + - - + + - - + + - - KD - - + + - - + + - - + + BasalStarvation Refeeding Supplemental Figure 1. Glucose-mediated regulation of ARRDC4 is dependent on MondoA in human skeletal myotubes. (A) (top) ARRDC4 and MLXIP (MondoA) mRNA levels were determined by qRT-PCR in human skeletal myotubes following deprivation of glucose at the indicated time (n=4). (bottom) Representative Western blot analysis of MondoA demonstrating the effect of glucose deprivation. *p<0.05 vs. 0h. (B) (top) ARRDC4 and MLXIP (MondoA) expression in human myotubes following a 6h glucose removal and refeeding at the times indicated (n=4). (bottom) Corresponding Western blot analysis. *p<0.05 vs Starvation 6h. (C) (top) Expression of ARRDC4 and MLXIP in human myotubes following deprivation and refeeding of glucose in the absence or presence of siRNA-mediated MondoA KD (n=4). (bottom) Corresponding Western blot analysis. *p<0.05 vs siControl. # p<0.05. § p<0.05. The data represents mean ± SD. All statistical significance determined by one-way ANOVA with Tukey multiple comparison post-hoc test.