Fluvial Range Expansion, Allopatry, and Parallel Evolution in a Danubian Snail Lineage (Neritidae: Theodoxus)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Trends of Aquatic Alien Species Invasions in Ukraine
Aquatic Invasions (2007) Volume 2, Issue 3: 215-242 doi: http://dx.doi.org/10.3391/ai.2007.2.3.8 Open Access © 2007 The Author(s) Journal compilation © 2007 REABIC Research Article Trends of aquatic alien species invasions in Ukraine Boris Alexandrov1*, Alexandr Boltachev2, Taras Kharchenko3, Artiom Lyashenko3, Mikhail Son1, Piotr Tsarenko4 and Valeriy Zhukinsky3 1Odessa Branch, Institute of Biology of the Southern Seas, National Academy of Sciences of Ukraine (NASU); 37, Pushkinska St, 65125 Odessa, Ukraine 2Institute of Biology of the Southern Seas NASU; 2, Nakhimova avenue, 99011 Sevastopol, Ukraine 3Institute of Hydrobiology NASU; 12, Geroyiv Stalingrada avenue, 04210 Kiyv, Ukraine 4Institute of Botany NASU; 2, Tereschenkivska St, 01601 Kiyv, Ukraine E-mail: [email protected] (BA), [email protected] (AB), [email protected] (TK, AL), [email protected] (PT) *Corresponding author Received: 13 November 2006 / Accepted: 2 August 2007 Abstract This review is a first attempt to summarize data on the records and distribution of 240 alien species in fresh water, brackish water and marine water areas of Ukraine, from unicellular algae up to fish. A checklist of alien species with their taxonomy, synonymy and with a complete bibliography of their first records is presented. Analysis of the main trends of alien species introduction, present ecological status, origin and pathways is considered. Key words: alien species, ballast water, Black Sea, distribution, invasion, Sea of Azov introduction of plants and animals to new areas Introduction increased over the ages. From the beginning of the 19th century, due to The range of organisms of different taxonomic rising technical progress, the influence of man groups varies with time, which can be attributed on nature has increased in geometrical to general processes of phylogenesis, to changes progression, gradually becoming comparable in in the contours of land and sea, forest and dimensions to climate impact. -

Shell Classification – Using Family Plates
Shell Classification USING FAMILY PLATES YEAR SEVEN STUDENTS Introduction In the following activity you and your class can use the same techniques as Queensland Museum The Queensland Museum Network has about scientists to classify organisms. 2.5 million biological specimens, and these items form the Biodiversity collections. Most specimens are from Activity: Identifying Queensland shells by family. Queensland’s terrestrial and marine provinces, but These 20 plates show common Queensland shells some are from adjacent Indo-Pacific regions. A smaller from 38 different families, and can be used for a range number of exotic species have also been acquired for of activities both in and outside the classroom. comparative purposes. The collection steadily grows Possible uses of this resource include: as our inventory of the region’s natural resources becomes more comprehensive. • students finding shells and identifying what family they belong to This collection helps scientists: • students determining what features shells in each • identify and name species family share • understand biodiversity in Australia and around • students comparing families to see how they differ. the world All shells shown on the following plates are from the • study evolution, connectivity and dispersal Queensland Museum Biodiversity Collection. throughout the Indo-Pacific • keep track of invasive and exotic species. Many of the scientists who work at the Museum specialise in taxonomy, the science of describing and naming species. In fact, Queensland Museum scientists -

Aquatic Molluscs of the Mrežnica River
NAT. CROAT. VOL. 28 No 1 99-106 ZAGREB June 30, 2019 original scientific paper / izvorni znanstveni rad DOI 10.20302/NC.2019.28.9 AQUATIC MOLLUSCS OF THE MREŽNICA RIVER Luboš Beran Nature Conservation Agency of the Czech Republic, Regional Office Kokořínsko – Máchův kraj Protected Landscape Area Administration, Česká 149, CZ–276 01 Mělník, Czech Republic (e-mail: [email protected]) Beran, L.: Aquatic molluscs of the Mrežnica River. Nat. Croat. Vol. 28, No. 1., 99-106, Zagreb, 2019. Results of a malacological survey of the Mrežnica River are presented. The molluscan assemblages of this river between the boundary of the Eugen Kvarternik military area and the inflow to the Korana River near Karlovac were studied from 2013 to 2018. Altogether 29 aquatic molluscs (19 gastropods, 10 bivalves) were found at 9 sites. Theodoxus danubialis, Esperiana esperi, Microcolpia daudebartii and Holandriana holandrii were dominant at most of the sites. The molluscan assemblages were very similar to assemblages documented during previous research into the Korana River. The populations of the endangered bivalves Unio crassus and Pseudanodonta complanata were recorded. An extensive population of another endangered gastropod Anisus vorticulus was found at one site. Physa acuta is the only non-native species confirmed in the Mrežnica River. Key words: Mollusca, Unio crassus, Anisus vorticulus, Mrežnica, faunistic, Croatia Beran, L.: Vodeni mekušci rijeke Mrežnice. Nat. Croat. Vol. 28, No. 1., 99-106, Zagreb, 2019. U radu su predstavljeni rezultati malakološkog istraživanja rijeke Mrežnice. Sastav mekušaca ove rijeke proučavan je na području od Vojnog poligona Eugen Kvarternik do njenog utoka u Koranu blizu Karlovca, u razdoblju 2013. -

A Conservation Palaeobiological Approach to Assess Faunal Response of Threatened Biota Under Natural and Anthropogenic Environmental Change
Biogeosciences, 16, 2423–2442, 2019 https://doi.org/10.5194/bg-16-2423-2019 © Author(s) 2019. This work is distributed under the Creative Commons Attribution 4.0 License. A conservation palaeobiological approach to assess faunal response of threatened biota under natural and anthropogenic environmental change Sabrina van de Velde1,*, Elisabeth L. Jorissen2,*, Thomas A. Neubauer1,3, Silviu Radan4, Ana Bianca Pavel4, Marius Stoica5, Christiaan G. C. Van Baak6, Alberto Martínez Gándara7, Luis Popa7, Henko de Stigter8,2, Hemmo A. Abels9, Wout Krijgsman2, and Frank P. Wesselingh1 1Naturalis Biodiversity Center, P.O. Box 9517, 2300 RA Leiden, the Netherlands 2Palaeomagnetic Laboratory “Fort Hoofddijk”, Faculty of Geosciences, Utrecht University, Budapestlaan 17, 3584 CD Utrecht, the Netherlands 3Department of Animal Ecology & Systematics, Justus Liebig University, Heinrich-Buff-Ring 26–32 IFZ, 35392 Giessen, Germany 4National Institute of Marine Geology and Geoecology (GeoEcoMar), 23–25 Dimitrie Onciul St., 024053 Bucharest, Romania 5Department of Geology, Faculty of Geology and Geophysics, University of Bucharest, Balcescu˘ Bd. 1, 010041 Bucharest, Romania 6CASP, West Building, Madingley Rise, Madingley Road, CB3 0UD, Cambridge, UK 7Grigore Antipa National Museum of Natural History, Sos. Kiseleff Nr. 1, 011341 Bucharest, Romania 8NIOZ Royal Netherlands Institute for Sea Research, Department of Ocean Systems, 1790 AB Den Burg, the Netherlands 9Department of Geosciences and Engineering, Delft University of Technology, Stevinweg 1, 2628 CN Delft, the Netherlands *These authors contributed equally to this work. Correspondence: Sabrina van de Velde ([email protected]) and Elisabeth L. Jorissen ([email protected]) Received: 10 January 2019 – Discussion started: 31 January 2019 Revised: 26 April 2019 – Accepted: 16 May 2019 – Published: 17 June 2019 Abstract. -
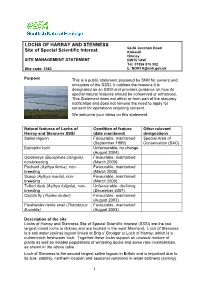
Lochs of Harray and Stenness Site of Special Scientific Interest (SSSI) Are the Two Largest Inland Lochs in Orkney and Are Located in the West Mainland
LOCHS OF HARRAY AND STENNESS 54-56 Junction Road Site of Special Scientific Interest Kirkwall Orkney SITE MANAGEMENT STATEMENT KW15 1AW Tel: 01856 875 302 Site code: 1083 E: [email protected] Purpose This is a public statement prepared by SNH for owners and occupiers of the SSSI. It outlines the reasons it is designated as an SSSI and provides guidance on how its special natural features should be conserved or enhanced. This Statement does not affect or form part of the statutory notification and does not remove the need to apply for consent for operations requiring consent. We welcome your views on this statement. Natural features of Lochs of Condition of feature Other relevant Harray and Stenness SSSI (date monitored) designations Saline lagoon Favourable, maintained Special Area of (September 1999) Conservation (SAC) Eutrophic loch Unfavourable, no change (August 2004) Goldeneye (Bucephala clangula), Favourable, maintained non-breeding (March 2000) Pochard (Aythya ferina), non- Favourable, maintained breeding (March 2000) Scaup (Aythya marila), non- Favourable, maintained breeding (March 2000) Tufted duck (Aythya fuligula), non- Unfavourable, declining breeding (December 2007) Caddis fly (Ylodes reuteri) Favourable, maintained (August 2003) Freshwater nerite snail (Theodoxus Favourable, maintained fluviatilis) (August 2003) Description of the site Lochs of Harray and Stenness Site of Special Scientific Interest (SSSI) are the two largest inland lochs in Orkney and are located in the west Mainland. Loch of Stenness is a salt water (saline) lagoon linked at Brig o’ Brodgar to Loch of Harray, which is a nutrient-rich freshwater loch. Together these lochs support an unusual mixture of plants as well as notable populations of wintering ducks and some rare invertebrates, as shown in the above table. -

The Marine and Brackish Water Mollusca of the State of Mississippi
Gulf and Caribbean Research Volume 1 Issue 1 January 1961 The Marine and Brackish Water Mollusca of the State of Mississippi Donald R. Moore Gulf Coast Research Laboratory Follow this and additional works at: https://aquila.usm.edu/gcr Recommended Citation Moore, D. R. 1961. The Marine and Brackish Water Mollusca of the State of Mississippi. Gulf Research Reports 1 (1): 1-58. Retrieved from https://aquila.usm.edu/gcr/vol1/iss1/1 DOI: https://doi.org/10.18785/grr.0101.01 This Article is brought to you for free and open access by The Aquila Digital Community. It has been accepted for inclusion in Gulf and Caribbean Research by an authorized editor of The Aquila Digital Community. For more information, please contact [email protected]. Gulf Research Reports Volume 1, Number 1 Ocean Springs, Mississippi April, 1961 A JOURNAL DEVOTED PRIMARILY TO PUBLICATION OF THE DATA OF THE MARINE SCIENCES, CHIEFLY OF THE GULF OF MEXICO AND ADJACENT WATERS. GORDON GUNTER, Editor Published by the GULF COAST RESEARCH LABORATORY Ocean Springs, Mississippi SHAUGHNESSY PRINTING CO.. EILOXI, MISS. 0 U c x 41 f 4 21 3 a THE MARINE AND BRACKISH WATER MOLLUSCA of the STATE OF MISSISSIPPI Donald R. Moore GULF COAST RESEARCH LABORATORY and DEPARTMENT OF BIOLOGY, MISSISSIPPI SOUTHERN COLLEGE I -1- TABLE OF CONTENTS Introduction ............................................... Page 3 Historical Account ........................................ Page 3 Procedure of Work ....................................... Page 4 Description of the Mississippi Coast ....................... Page 5 The Physical Environment ................................ Page '7 List of Mississippi Marine and Brackish Water Mollusca . Page 11 Discussion of Species ...................................... Page 17 Supplementary Note ..................................... -

A Late Pleistocene Gastropod Fauna from the Northern Caspian Sea with Implications for Pontocaspian Gastropod Taxonomy
A peer-reviewed open-access journal ZooKeys 770: 43–103 (2018)A late Pleistocene gastropod fauna from the northern Caspian Sea... 43 doi: 10.3897/zookeys.770.25365 RESEARCH ARTICLE 4 ZooKeys http://zookeys.pensoft.net Launched to accelerate biodiversity research A late Pleistocene gastropod fauna from the northern Caspian Sea with implications for Pontocaspian gastropod taxonomy Thomas A. Neubauer1,2, Sabrina van de Velde2, Tamara Yanina3, Frank P. Wesselingh2 1 Department of Animal Ecology and Systematics, Justus Liebig University, Heinrich-Buff-Ring 26–32 IFZ, 35392 Giessen, Germany 2 Naturalis Biodiversity Center, P.O. Box 9517, 2300 RA Leiden, The Netherlands 3 Moscow State University, Faculty of Geography, Leninskie Gory, 1, 119991 Moscow, Russia Corresponding author: Thomas A. Neubauer ([email protected]) Academic editor: M. Haase | Received 29 March 2018 | Accepted 20 May 2018 | Published 4 July 2018 http://zoobank.org/4D984FDD-9366-4D8B-8A8E-9D4B3F9B8EFB Citation: Neubauer TA, van de Velde S, Yanina T, Wesselingh FP (2018) A late Pleistocene gastropod fauna from the northern Caspian Sea with implications for Pontocaspian gastropod taxonomy. ZooKeys 770: 43–103. https://doi. org/10.3897/zookeys.770.25365 Abstract The present paper details a very diverse non-marine gastropod fauna retrieved from Caspian Pleistocene deposits along the Volga River north of Astrakhan (Russia). During time of deposition (early Late Pleis- tocene, late Khazarian regional substage), the area was situated in shallow water of the greatly expanded Caspian Sea. The fauna contains 24 species, of which 16 are endemic to the Pontocaspian region and 15 to the Caspian Sea. -

A Stable Temperature May Favour Continuous Reproduction by Theodoxus fluviatilis and Explain Its High Densities in Some Karstic Springs
Limnetica, 29 (2): x-xx (2011) Limnetica, 31 (1): 129-140 (2012). DOI: 10.23818/limn.31.12 c Asociaci´on Ib´erica de Limnologı´a, Madrid. Spain. ISSN: 0213-8409 A stable temperature may favour continuous reproduction by Theodoxus fluviatilis and explain its high densities in some karstic springs Manuel A. S. Grac¸a∗,Sonia´ R. Q. Serra and Veronica´ Ferreira IMAR-CMA and Department of Life Sciences, University of Coimbra, PO Box 3046, 3001-401 Coimbra, Portugal. ∗ Corresponding author: [email protected] 2 Received: 30/5/2011 Accepted: 27/12/2011 ABSTRACT A stable temperature may favour continuous reproduction by Theodoxus fluviatilis and explain its high densities in some karstic springs Theodoxus fluviatilis is a common gastropod in many karstic springs in central Portugal. We investigated the possible reasons for the near-total restriction of this species to these springs. We first determined the spatial distribution of the species within a spring (Anc¸os) and related the densities at sampling-patch scales to selected physical and chemical variables. We then determined the densities at several locations downstream from the spring and related these densities to selected physical and chemical variables. Finally, we assessed the population dynamics of the gastropod in the spring. In the spring, T. fluviatilis was more abundant in shallow areas with a rapid current and cobble-boulder substrates. In June-July 2006, the mean densities of T. fluviatilis in the spring varied from ∼ 10 to ∼ 9000 individuals m–2 but decreased to zero 3800 m downstream. The physical and chemical changes along the stretch studied were minor; no significant correlations (Spearman rank correlation; p > 0.05) were observed between the gastropod abundances and the measured environmental variables or the PCA axes. -

The Morphological Plasticity of Theodoxus Fluviatilis (Linnaeus, 1758) (Mollusca: Gastropoda: Neritidae)
Correspondence ISSN 2336-9744 (online) | ISSN 2337-0173 (print) The journal is available on line at www.ecol-mne.com The morphological plasticity of Theodoxus fluviatilis (Linnaeus, 1758) (Mollusca: Gastropoda: Neritidae) PETER GLÖER 1 and VLADIMIR PEŠI Ć2 1 Biodiversity Research Laboratory, Schulstraße 3, D-25491 Hetlingen, Germany. E-mail: [email protected] 2 Department of Biology, Faculty of Sciences, University of Montenegro, Cetinjski put b.b., 81000 Podgorica, Montenegro. E-mail: [email protected] Received 19 January 2015 │ Accepted 3 February 2015 │ Published online 4 February 2015. Theodoxus fluviatilis is a widely distributed species, ranging from the Ireland (Lucey et al . 1992) to Iran (Glöer & Peši ć 2012). The records from Africa were considered as doubtful (Brown 2002), but recently the senior author found this species in Algeria (Glöer unpublished record). The species prefers the lowlands (in Switzerland up to 275 m a.s.l., Turner et al . 1998), and calcium-rich waters, living on hard benthic substrates, typically rocks. On the territory of the Central and Eastern Balkans three species are present (Markovi ć et al . 2014): Theodoxus fluviatilis (Linnaeus, 1758), T. danubialis (C. Pfeiffer, 1828) and T. transversalis (C.Pfeiffer, 1828). For Montenegro, Wohlberedt (1909), Karaman & Karaman (2007), Glöer & Peši ć (2008), and Peši ć & Glöer (2013) listed only Theodoxus fluviatilis . Figures 1-4. The operculum. 1: Theodoxus fluviatilis (Linnaeus, 1758); 2: T. danubialis (C. Pfeiffer, 1828); 3-4: Rib shield of T. fluviatilis . Abbreviations: ca = callus, eo = embryonic operculum, la = left adductor, p = peg, r = rib, ra = right adductor, rp = rib pit, rs = rib shield. The shell of adults of Theodoxus fluviatilis is 4.5–6.5 mm high and 6–9 mm broad, exceptionally up to 13 mm, and consists of 3–3.5 whorls, including protoconch, with a usually low spire. -

Fossil Flora and Fauna of Bosnia and Herzegovina D Ela
FOSSIL FLORA AND FAUNA OF BOSNIA AND HERZEGOVINA D ELA Odjeljenje tehničkih nauka Knjiga 10/1 FOSILNA FLORA I FAUNA BOSNE I HERCEGOVINE Ivan Soklić DOI: 10.5644/D2019.89 MONOGRAPHS VOLUME LXXXIX Department of Technical Sciences Volume 10/1 FOSSIL FLORA AND FAUNA OF BOSNIA AND HERZEGOVINA Ivan Soklić Ivan Soklić – Fossil Flora and Fauna of Bosnia and Herzegovina Original title: Fosilna flora i fauna Bosne i Hercegovine, Sarajevo, Akademija nauka i umjetnosti Bosne i Hercegovine, 2001. Publisher Academy of Sciences and Arts of Bosnia and Herzegovina For the Publisher Academician Miloš Trifković Reviewers Dragoljub B. Đorđević Ivan Markešić Editor Enver Mandžić Translation Amra Gadžo Proofreading Amra Gadžo Correction Sabina Vejzagić DTP Zoran Buletić Print Dobra knjiga Sarajevo Circulation 200 Sarajevo 2019 CIP - Katalogizacija u publikaciji Nacionalna i univerzitetska biblioteka Bosne i Hercegovine, Sarajevo 57.07(497.6) SOKLIĆ, Ivan Fossil flora and fauna of Bosnia and Herzegovina / Ivan Soklić ; [translation Amra Gadžo]. - Sarajevo : Academy of Sciences and Arts of Bosnia and Herzegovina = Akademija nauka i umjetnosti Bosne i Hercegovine, 2019. - 861 str. : ilustr. ; 25 cm. - (Monographs / Academy of Sciences and Arts of Bosnia and Herzegovina ; vol. 89. Department of Technical Sciences ; vol. 10/1) Prijevod djela: Fosilna flora i fauna Bosne i Hercegovine. - Na spor. nasl. str.: Fosilna flora i fauna Bosne i Hercegovine. - Bibliografija: str. 711-740. - Registri. ISBN 9958-501-11-2 COBISS/BIH-ID 8839174 CONTENTS FOREWORD ........................................................................................................... -

The Fossil Record of Shell-Breaking Predation on Marine Bivalves and Gastropods
Chapter 6 The Fossil Record of Shell-Breaking Predation on Marine Bivalves and Gastropods RICHARD R. ALEXANDER and GREGORY P. DIETL I. Introduction 141 2. Durophages of Bivalves and Gastropods 142 3. Trends in Antipredatory Morphology in Space and Time .. 145 4. Predatory and Non-Predatory Sublethal Shell Breakage 155 5. Calculation ofRepair Frequencies and Prey Effectiveness 160 6. Prey Species-, Size-, and Site-Selectivity by Durophages 164 7. Repair Frequencies by Time, Latitude, and Habitat.. 166 8. Concluding Remarks 170 References 170 1. Introduction Any treatment of durophagous (shell-breaking) predation on bivalves and gastropods through geologic time must address the molluscivore's signature preserved in the victim's skeleton. Pre-ingestive breakage or crushing is only one of four methods of molluscivory (Vermeij, 1987; Harper and Skelton, 1993), the others being whole organism ingestion, insertion and extraction, and boring. Other authors in this volume treat the last behavior, whereas whole-organism ingestion, and insertion and extraction, however common, are unlikely to leave preservable evidence. Bivalve and gastropod ecologists and paleoecologists reconstruct predator-prey relationships based primarily on two, although not equally useful, categories of pre-ingestive breakage, namely lethal and sublethal (repaired) damage. Peeling crabs may leave incriminating serrated, helical RICHARD R. ALEXANDER • Department of Geological and Marine Sciences, Rider University, Lawrenceville, New Jersey, 08648-3099. GREGORY P. DIETL. Department of Zoology, North Carolina State University, Raleigh, North Carolina, 27695-7617. Predator-Prey Interactions in the Fossil Record, edited by Patricia H. Kelley, Michal Kowalewski, and Thor A. Hansen. Kluwer Academic/Plenum Publishers, New York, 2003. 141 142 Chapter 6 fractures in whorls of high-spired gastropods (Bishop, 1975), but unfortunately most lethal fractures are far less diagnostic of the causal agent and often indistinguishable from abiotically induced, taphonomic agents ofshell degradation. -

From the Iberian Peninsula
Ecologica Montenegrina 18: 133-137 (2018) This journal is available online at: www.biotaxa.org/em On the identity of Neritina baetica Lamarck, 1822 and Nerita meridionalis Philippi, 1836 (Gastropoda: Neritidae) from the Iberian Peninsula PETER GLÖER Biodiversity Research Laboratory, Schulstraße 3, D-25491 Hetlingen, Germany, e-mail: [email protected] Received 2 August 2018 │ Accepted by V. Pešić: 29 August 2018 │ Published online 1 September 2018. Abstract When Mermod (1953) depicted the types of Theodoxus baeticus of Lamarck’s collection his photo did not display a pseudo-apophysis (peg). This led recent authors believe, that Th. baeticus is a synonym of Th. fluviatilis and Theodoxus meridionalis is the valid species (Alba et al. 2016: 55). In this paper I studied the syntypes of Th. baeticus as well as the syntypes of Th. meridionalis and demonstrated that in Th. baeticus a pseudo-apophysis exists and the opercula of Th. baeticus and Th. meridionalis are similar. Thus Th. baeticus is a good species and Th. meridionalis is a younger synonym of it. Therefore I can conclude that three Theodoxus spp., i.e., Th. fluviatilis, Th. baeticus and Th. valentinus occur in the Iberian Peninsula. Key words: Theodoxus baeticus, Theodoxus meridionalis, Iberian Peninsula, syntypes. Introduction Many species have formerly been described on the basis of shell morphology and especially on the patterns or the color of the shells. Many of these nominal taxa belong at least to Theodoxus fluviatilis. For instance in Central Europe Th. fluviatilis has patterns of drop shape, while it has in Spain zigzag lines, and in the Balkans we find a combination of both patterns (Glöer & Pešić 2015).