CTRI Trial Data
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Coimbatore Commissionerate Jurisdiction
Coimbatore Commissionerate Jurisdiction The jurisdiction of Coimbatore Commissionerate will cover the areas covering the entire Districts of Coimbatore, Nilgiris and the District of Tirupur excluding Dharapuram, Kangeyam taluks and Uthukkuli Firka and Kunnathur Firka of Avinashi Taluk * in the State of Tamil Nadu. *(Uthukkuli Firka and Kunnathur Firka are now known as Uthukkuli Taluk). Location | 617, A.T.D. STR.EE[, RACE COURSE, COIMBATORE: 641018 Divisions under the jurisdiction of Coimbatore Commissionerate Sl.No. Divisions L. Coimbatore I Division 2. Coimbatore II Division 3. Coimbatore III Division 4. Coimbatore IV Division 5. Pollachi Division 6. Tirupur Division 7. Coonoor Division Page 47 of 83 1. Coimbatore I Division of Coimbatore Commissionerate: Location L44L, ELGI Building, Trichy Road, COIMBATORT- 641018 AreascoveringWardNos.l to4,LO to 15, 18to24and76 to79of Coimbatore City Municipal Corporation limit and Jurisdiction Perianaickanpalayam Firka, Chinna Thadagam, 24-Yeerapandi, Pannimadai, Somayampalayam, Goundenpalayam and Nanjundapuram villages of Thudiyalur Firka of Coimbatore North Taluk and Vellamadai of Sarkar Samakulam Firka of Coimbatore North Taluk of Coimbatore District . Name of the Location Jurisdiction Range Areas covering Ward Nos. 10 to 15, 20 to 24, 76 to 79 of Coimbatore Municipal CBE Corporation; revenue villages of I-A Goundenpalayam of Thudiyalur Firka of Coimbatore North Taluk of Coimbatore 5th Floor, AP Arcade, District. Singapore PIaza,333 Areas covering Ward Nos. 1 to 4 , 18 Cross Cut Road, Coimbatore Municipal Coimbatore -641012. and 19 of Corporation; revenue villages of 24- CBE Veerapandi, Somayampalayam, I-B Pannimadai, Nanjundapuram, Chinna Thadagam of Thudiyalur Firka of Coimbatore North Taluk of Coimbatore District. Areas covering revenue villages of Narasimhanaickenpalayam, CBE Kurudampalayam of r-c Periyanaickenpalayam Firka of Coimbatore North Taluk of Coimbatore District. -
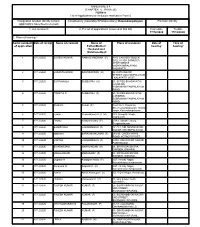
ANNEXURE 5.8 (CHAPTER V , PARA 25) FORM 9 List of Applications For
ANNEXURE 5.8 (CHAPTER V , PARA 25) FORM 9 List of Applications for inclusion received in Form 6 Designated location identity (where Constituency (Assembly/£Parliamentary): Kavundampalayam Revision identity applications have been received) 1. List number@ 2. Period of applications (covered in this list) From date To date 17/12/2020 17/12/2020 3. Place of hearing * Serial number$ Date of receipt Name of claimant Name of Place of residence Date of Time of of application Father/Mother/ hearing* hearing* Husband and (Relationship)# 1 17/12/2020 SURESHKUMAR RAMACHANDIRAN (F) A103 CAUVERY BLOCK, SREEVATSA SANKARA APARTMENTS VAZHIYAMPALAYAM, KALAPATTI, , 2 17/12/2020 VASANTHAMANI SOUNDAPPAN (H) 22, RATHINAGRI STREET,VALIYAMPALAYAM , KALAPATTI WEST, , 3 17/12/2020 SURIYAKALA SUBBURAJ (H) 21, SHREE BHAGAVATHI GARDENS, SUBRAMANIYAMPALAYAM ROAD, , 4 17/12/2020 PREETHI S SUBBURAJ (F) 21, SHREE BHAGAVATHI GARDENS, SUBRAMANIYAMPALAYAM ROAD, , 5 17/12/2020 Shalini K Kumar (F) Door No 1, Poornima Street,Edayarpalayam, Ganthi nagar, Kavundampalayam, , 6 17/12/2020 Eswari Krishnakumar P G (H) 139, Sampath Nagar, Vilankurichi , , 7 17/12/2020 Prabhu Narayanasamy (F) 2/163, Gandhi Colony, Sengalipalayam, , 8 17/12/2020 BHIMSINGH LAKSHMANAN (F) 131/1-1, SRI SENTHUR SRI NAGAR, DEVAMPALAYAM, , 9 17/12/2020 siddharth SARAVANAKUMAR (F) 28/38E, GREEN WAYS ROAD, KOTAGIRI POST, , 10 17/12/2020 VINITHA LAKSHMANAN (F) 5/409, VINAYAGAR NAGAR, PANNIMADAI, , 11 17/12/2020 SIVARANJANI MAHALINGAM (H) 28, SRI RAGAVENDRA GARDEN, IDIKARAI, , 12 17/12/2020 MAHALINGAM MADASAMY -

Manchester-Cottoncity-Brochure.Pdf
MANCHESTER Cottoncity Prestigious Legacy for your loved one... About Coimbare Coimbatore, popularly known as “The Manchester of South India” is renowned for its pleasant climatic condition, landscape and its hospitality. Coimbatore - a major industrial city boasts of a vibrant growing economy and a good social infrastructure. The smart city stands with pride because of quality educational institutions and world class health care services. Manchesr Cotnci Cotton City Developers is all set to add yet another masterpiece- MANCHESTER COTTONCITY to its existing and highly acclaimed Manchester brand of properties. MANCHESTER COTTONCITY has 72 exquisitely designed and luxuriant Villas with impeccable specifications and it comes with an exceptional location advantage too. The Gated Community falls well within the city limits yet free from the noise and clutter, thus paving the way to lead a serene and peaceful living. Large living spaces, club house and modern amenities with contemporary architectural designs, landscaping with good infrastructure makes it the perfect destination for your family. MANCHESTER COTTONCITY is strategically located at Vellakinar, on Thudiyalur- Saravanampatti Road, Coimbatore and is very close to IT park, shopping malls, hospitals and educational institutions. Click here for the Walk-through Presentation www.youtube.com/watch?v=LIXd0NTCEtg Where Community becomes family Amenities Club House with Gymnasium Landscaped Garden Pool Table Swimming Pool Table Tennis / Badminton / Children’s Park Conference Hall Foosball Table Basketball Court Cable TV / Internet Intercom Provided Generator Recreation Rooms Provision Tennis Court Cricket Net RO Treatment Plant Visitors Car Parking Designed as per CCTV Camera Gated Community with Convenience Store Vasthu Round the Clock Security. Sample Floor Plan Ground Floor First Floor Built Up Area : 2,502 sqft Carpet Area : 1,534 sqft Land Area : 6.7 - 7.3 cents Terrace Specifications Super Structure : RCC framed structure with isolated footing foundation designed Intercom will be provided in living room. -
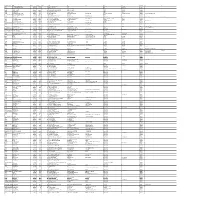
SGL- UNPAID SHAREHOLDER LIST AS on 30-09-2019.Xlsx
FOLIO-DEMAT ID NAME DWNO NETDIV MICR AD1 AD2 AD3 AD4 CITY PINCOD JH1 JH2 U00481 UTTAM KUMAR MALHOTRA 19200002 2000.00 1487 61, SRI ARABINDA ROAD SALKIA HOWRAH HOWRAH 6 A01126 AHUJA REFRIGERATION (P) LTD 19200004 2000.00 1489 1290, KATRA DHULIA CH-CH DELHI NEW DELHI 110006 S02633 SUSHMA WADHWA 19200006 2000.00 1491 29, INDRA VIHAR NEAR B B M DEPOT DELHI NEW DELHI 110009 A00436 ANIL KUMAR GUPTA 19200009 11000.00 1494 C/O. BALGOVIND BRINDA PRASAD 51/53, NAYA GANJ KANPUR KANPUR 208001 S00441 SAPNA RANI JAIN 19200010 2740.00 1495 77/43-A COOLIE BAZAR KANPUR KANPUR 208001 A00489 ANURAG GUPTA 19200011 5011.00 1496 113/59, IST FLOOR SWAROOP NAGAR KANPUR KANPUR 208002 K00538 KOKILABEN PRAVINBHAI PATEL 19200022 1740.00 1507 C/3, SHREEJI COLONY BEHIND PRAGATI MANDAL MAHADEV AREA VALLABH VIDYANAGAR VALLABH VIDYANAGAR 388120 PRAVINBHAI FULABHAI PATEL 1301670000343745 JAYNARAYAN VALLABHBHAI PATEL 19200023 3480.00 1508 NAVA FALIA MUWADA JHALOD 389170 000729 DAVALBEN VIRJIBHAI VAGHANI 19200025 16440.00 1510 12-A, SHREE RAMNAGAR SOCIETY HIRABAUG VARACHHA ROAD SURAT 395006 D00384 DHRUTI V SHAH 19200027 4000.00 1512 C/O M/S VARJIVANDAS & CO 174 DR D N ROAD HORNBY BUILDING FORT MUMBAI MUMBAI 400001 VINOD V SHAH RANJAN V SHAH S01754 SUDHA RAVI 19200029 2740.00 1514 C/O.MR. RAVI KUMAR M A STANDARD CHARTERED BANK GLOBAL MARKETS 90,M G ROAD, FORT MUMBAI MUMBAI 400001 K00436 KAPIL KUMAR SACHDEV 19200040 5200.00 1525 NAVJIVAN KUTIR 6 TH FLOOR 31 A ALTAMOUNT ROAD MUMBAI MUMBAI 400026 K01443 KAUSHAL A SHAH 19200042 2000.00 1527 C-114, DADAR PASCHIM APARTMENTS KD MARG OPP. -

Coimbatore Educational District
Coimbatore Educational District TET Application Sales Centre Sl.No Name of the School 1 GOVT HR.SEC.SCHOOL, S.N.PALAYAM, COIMBATORE - 641016. CORPORATION HR.SEC.SCHOOL, R.S.PURAM (GIRLS), COIMBATORE - 2 641 002 CORPORATION HR.SEC.SCHOOL, RANGANATHAPURAM (GIRLS), 3 COIMBATORE - 641 009 CORPORATION HR.SEC.SCHOOL, R.S.PURAM (BOYS), COIMBATORE - 4 641 002 CITY CORPORATION HR.SEC.SCHOOL, V.H.ROAD, COIMBATORE - 5 641001 6 CORPORATION HR.SEC.SCHOOL, VADAKOVAI, COIMBATORE - 641012 CORPORATION HR.SEC.SCHOOL, VENKITTAPURAM, COIMBATORE - 7 641013 S.R.P AMMANIAMMAL CORPORATION HR.SEC.SCHOOL, R.S.PURAM, 8 (GIRLS), COIMBATORE - 641002 9 GOVT HR.SEC.SCHOOL, ONDIPUDUR (GIRLS), COIMBATORE - 641016 10 GOVT HR.SEC.SCHOOL, ONDIPUDUR (BOYS) COIMBATORE - 641016 11 GOVT HR.SEC.SCHOOL, VELLALORE COIMBATORE - 641111 GOVT HR.SEC.SCHOOL, SINGANALLUR (GIRLS), COIMBATORE - 12 641005 CORPORATION HR.SEC.SCHOOL, RAMAKRISHNAPURAM (GIRLS), 13 COIMBATORE - 641 045 CORPORATION HR.SEC.SCHOOL, RAMANATHAPURAM (BOYS), 14 COIMBATORE - 641 045 15 GOVT HR.SEC.SCHOOL, RAJA STREET, COIMBATORE - 641001 16 GOVT HR.SEC.SCHOOL, SUNDAKAMUTHUR, COIMBATORE - 641010. GOVT HR.SEC.SCHOOL, KULATHUPALAYAM, COIMBATORE - 17 641042 CORPORATION HR.SEC.SCHOOL, SELVAPURAM (BOYS), COIMBATORE 18 - 641026 CORPORATION HR.SEC.SCHOOL, OPPANAKARA STREET, (GIRLS), 19 COIMBATORE - 641001 CORPORATION HR.SEC.SCHOOL, OKKILIYAR COLONY, COIMBATORE - 20 641001 21 GOVT HR.SEC.SCHOOL, S.S.KULAM, COIMBATORE - 641107 Sl.No Name of the School 22 GOVT HR.SEC.SCHOOL, VELLAMADAI, COIMBATORE - 641110 23 GOVT HR.SEC.SCHOOL, -

Manchester Mayberry
https://www.propertywala.com/manchester-mayberry-coimbatore Manchester Mayberry - Thudialur, Coimbatore Residential plots for sale @vellakinar under city limit DTCP approved projects Manchester Mayberry by Manchester Properties at the very prime location of Thudialur in Coimbatore offers residential project that host land and 2 and 3 bhk villas in various sizes. Project ID: J919012331 Builder: Manchester Properties Location: Manchester Mayberry, Vellakinar Village, Thudialur, Coimbatore - 641029 (Tamil Nadu) Completion Date: Dec, 2017 Status: Started Description Manchester Mayberry by Manchester Properties at the very prime location of Vellakinar Village, Thudialur in Coimbatore offers residential project that host spacious land and 2 and 3 bhk villas in the size ranges in between 900 to 1670 sqft. It offers amenities like Grocery shop, Car parking and Villa,Land are Vastu complaint. The project is strategically located and have all the basic utilities are nearby. Amenities: Grocery Shop Vastu Compliant 24x7 Security Gated Community 24/7 Water Supply Children's Play Area Car Parking Internal Street Lights Fire Fighting Systems Manchester Properties is a leading real estate developer in and around the regions of Coimbatore. It was established with an aim to offer affordable housing solutions to the customers and has successfully delivered numerous prestigious projects. Company’s portfolio encompasses apartments, villas and houses. It is driven by a team of highly experienced and skilled personnel who work hard to come up with exceptional -

Abatment of Traffic in Railway Crossing Area by Road Under Bridge
IJMTES | International Journal of Modern Trends in Engineering and Science ISSN: 2348-3121 ABATMENT OF TRAFFIC IN RAILWAY CROSSING AREA BY ROAD UNDER BRIDGE S.Premalatha1, BA.Rathina Lakshmi2, D.Thokai Kasthuri2, M.Vijaya Rani2, S.Vinothini2 1(Asst professor, Dept of Civil Engg, Avinashilingam Institute for women, Coimbatore-641108, [email protected]) 2(B.E Student, Dept of Civil Engg, Avinashilingam Institute for women, Coimbatore-641108, [email protected]) ______________________________________________________________________________________________________ Abstract— Transportation is necessary in economic, social, political and cultural manner. The number of vehicles in India has been rapidly enlarging where Coimbatore ranks next to Chennai as a developing city of Tamil Nadu. Coimbatore is a well-developed city with heavy traffic, but its road infrastructure are poorly maintained making traffic congestion a major problems in city. The problem is identified in the Villakenaru pirivu in the place of THUDIYALUR-SARAVANAMPATTI road in COIMBATORE, TAMIL NADU. In order to reduce road block, the maximum solution is increasing its infrastructure. This location consists more of educational and commercial buildings with heavy traffic flow. The circumstance of flyover needs more space, we have proposed a Road under Bridge (RUB), to reduce the traffic congestion in that place. RUB is designed as Reinforced Cement Concrete BOX. The proposed structure is designed manually and analysed by using STAAD.PRO software. Keywords— Road Under Bridge; Precast RCC Box _________________________________________________________________________________________________________________ 1. INTRODUCTION traffic, parking and accidents. The main entrance is also by the public buses often passing from outside the city to Transportation plays a vital role in the trade between the another part, creates problems for the regular traffic people, which develops the civilization and also the moments. -
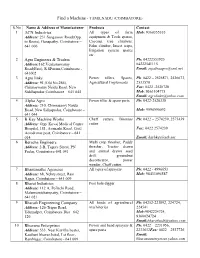
List of Manufacturers in Coimbatore
Find a Machine - TAMILNADU (COIMBATORE) S.No Name & Address of Manufacturer Products Contact 1 ACN Industries All types of farm Mob: 9360355333 Address: 231 Sanganoor Road(Opp. equipment & Tools spares, to Roots), Ganapathy, Coimbatore – Coconut tree climbers, 641 006 Palm climber, Insect traps, Irrigation system spares etc. 2 Agro Engineers & Traders Ph: 04222553925 Address:162,Venkataswamy 04222541115 Road(East), R.SPuram,Coimbatore - Email: [email protected] 641002 3 Agro links Power tillers, Spares, Ph: 0422 – 2526871, 2526673, Address: 91,(Old No.286), Agricultural Implements 2533570 Chinnaswamy Naidu Road, New Fax: 0422 -2520728 Siddhapudur,Coimbatore – 641 044 Mob: 9363104775 Email: [email protected] 4 Alpha Agro Power tiller & spare parts Ph: 0422-2520338 Address: 210, Chinnasamy Naidu Road, New Sidhapudur, Coimbatore – Mob: 9994990092 641 044 5 B Kay Machine Works Chaff cutters, Biomass Ph: 0422 – 2574239, 2573439 Address: Opp. Kovai Medical Centre cutter Hospital, 181, Avanashi Road, Civil Fax: 0422 2574239 Aerodrome post, Coimbatore – 641 014. Email: [email protected] 6 Beracha Engineers Multi crop thresher, Paddy Address: 2-B, Tagore Street, PN thresher, Tractor drawn Pudur, Coimbatore-641 041 and animal drawn seed drill, groundnut decorticator, power weeder, Chaff cutter. 7 Bhanumathe Agencies All types of sprayers Ph: 0422 - 4996653 Address: 68, Nehru street, Ram Mob: 98433094587 Nagar, Coimbatore – 641 009 8 Bharat Industries Post hole digger Address: 112 A, Pollachi Road, Malumamichampatty, Coimbatore – 641 021 9 Bharath Engineering Company All kinds of agricultural Ph: 04252-223892, 224724, Address: 126-Tripur Road, machineries 224541 Udumalpet, Coimbatore Dist– 642 Mob:9842224724, 126 9360024724 Email:[email protected] 10 Bhuvana Enterprises Power and hand sprayers & Ph: 0422 – 2231956, Address: 533, Near Kavitha heater, spare parts 2233632Fax: 0422 – 2537726 Kasthuri bhavan hotel, I st floor, Email: RamNagar, Coimbatore - 641 009 bhuvanaenterprises.yahoo.com 11 Bull Agro Implements. -

List of Town Panchayats Name in Tamil Nadu Page 1 District Code
List of Town Panchayats Name in Tamil Nadu Sl. No. District Code District Name Town Panchayat Name 1 1 KANCHEEPURAM ACHARAPAKKAM 2 1 KANCHEEPURAM CHITLAPAKKAM 3 1 KANCHEEPURAM EDAKALINADU 4 1 KANCHEEPURAM KARUNGUZHI 5 1 KANCHEEPURAM KUNDRATHUR 6 1 KANCHEEPURAM MADAMBAKKAM 7 1 KANCHEEPURAM MAMALLAPURAM 8 1 KANCHEEPURAM MANGADU 9 1 KANCHEEPURAM MEENAMBAKKAM 10 1 KANCHEEPURAM NANDAMBAKKAM 11 1 KANCHEEPURAM NANDIVARAM - GUDUVANCHERI 12 1 KANCHEEPURAM PALLIKARANAI 13 1 KANCHEEPURAM PEERKANKARANAI 14 1 KANCHEEPURAM PERUNGALATHUR 15 1 KANCHEEPURAM PERUNGUDI 16 1 KANCHEEPURAM SEMBAKKAM 17 1 KANCHEEPURAM SEVILIMEDU 18 1 KANCHEEPURAM SHOLINGANALLUR 19 1 KANCHEEPURAM SRIPERUMBUDUR 20 1 KANCHEEPURAM THIRUNEERMALAI 21 1 KANCHEEPURAM THIRUPORUR 22 1 KANCHEEPURAM TIRUKALUKUNDRAM 23 1 KANCHEEPURAM UTHIRAMERUR 24 1 KANCHEEPURAM WALAJABAD 25 2 TIRUVALLUR ARANI 26 2 TIRUVALLUR CHINNASEKKADU 27 2 TIRUVALLUR GUMMIDIPOONDI 28 2 TIRUVALLUR MINJUR 29 2 TIRUVALLUR NARAVARIKUPPAM 30 2 TIRUVALLUR PALLIPATTU 31 2 TIRUVALLUR PONNERI 32 2 TIRUVALLUR PORUR 33 2 TIRUVALLUR POTHATTURPETTAI 34 2 TIRUVALLUR PUZHAL 35 2 TIRUVALLUR THIRUMAZHISAI 36 2 TIRUVALLUR THIRUNINDRAVUR 37 2 TIRUVALLUR UTHUKKOTTAI Page 1 List of Town Panchayats Name in Tamil Nadu Sl. No. District Code District Name Town Panchayat Name 38 3 CUDDALORE ANNAMALAI NAGAR 39 3 CUDDALORE BHUVANAGIRI 40 3 CUDDALORE GANGAIKONDAN 41 3 CUDDALORE KATTUMANNARKOIL 42 3 CUDDALORE KILLAI 43 3 CUDDALORE KURINJIPADI 44 3 CUDDALORE LALPET 45 3 CUDDALORE MANGALAMPET 46 3 CUDDALORE MELPATTAMPAKKAM 47 3 CUDDALORE PARANGIPETTAI -

Coimbatore Corporation (Under Rajiv Awas Yojana) 2013 - 2022
SLUM FREE CITY PLAN OF ACTION - COIMBATORE CORPORATION (UNDER RAJIV AWAS YOJANA) 2013 - 2022 2013 Prepared By Submitted to National Institute of Tamil Nadu Slum Clearance Board, Technical Teachers Chennai Training and Research, Chennai – 600 113 CONTENTS Chapter 1. Overview 1.1 Introduction 01 1.2 Indian Scenario 02 1.3 Understanding Slums 05 1.4 Schemes to Alleviate Urban Poverty 07 1.4.1 Vision of Slum Free India: Launch of Rajiv Awas Yojana (RAY) 07 1.5 Objective and Scope of the Project 10 Chapter 2. Slum Survey and Investigation 2.1 City an Overview 11 2.1.1 History 11 2.1.2 Geography and Soil 12 2.1.3 Climate & Rainfall 14 2.1.4 Rapid Growth of the City 14 2.2 Overview of the ULB 15 2.3 Land Use of the Coimbatore 18 2.4 Diagnostic assessment of slums 19 2.5 Surveys, Investigations and Consultations 20 2.5.1 Slums not covered under RAY – Developed slums 20 2.5.2 Slums not covered under RAY – Opposition from Slums 23 2.5.3 Surveyed slums under RAY 27 2.6 Methodology 30 2.7 Socio Economic Survey 32 2.7.1 Stakeholder Consultation 32 2.8 Categorization of Slums based on Tenability Analysis 48 2.8.1 Tenable Slum 48 2.8.2 Untenable Slum 48 2.8.3 Semi-tenable Slum 49 2.9 Tenure 52 Chapter 3. Assessment of Present Status of Slums 3.1 Introduction 60 3.1.1 Vulnerability Parameters 60 i 3.1.2 Infrastructure Deficiency 61 3.2 Vulnerability Analysis 61 3.2.1 BPL Analysis 61 3.2.2 SC/ST Population Analysis 62 3.2.3 Structural Type Analysis 63 3.3 Infrastructure Deficiency Analysis 65 3.3.1 Water Supply 65 3.3.2 Individual Toilet facility 66 3.3.3 Storm Drainage facility 67 3.3.4 Solid waste disposal facility 67 3.3.5 Street Light facility 68 3.3.6 Road facility 69 3.4 Deficiency Matrix 70 3.4.1 Tenable Slum Classification based on Deficiency Matrix 76 3.4.2 Untenable Slum Prioritization 81 Chapter 4. -

ECONOMICS FINANCE 2018 Visit to Financial Institutions
ECONOMICS FINANCE 2018 Visit to Financial Institutions Batch 2017-19, finance students visited commercial banks in teamson February 17, 2018as part of their Commercial Banking course. South Indian Bank , Sungam branch; Axis Bank, R.S. Puram branch; Indian Bank Town hall; Indian Bank, Siddhapudhur; Central Bank, Thadagam Branch, HSBC, Race Course branch; Catholic Syrian Bank, Gandhipuram branch, Axis Bank, Saravanampatti Branch . The teams interacted with bank officials to get to know the modus operandi of bank and collected data on various aspects like types of retail loans, Credit evaluation- credit analysis-credit scoring and consumer credit regulations, BASEL norms, Loan Pricing, Objectives, models, CIBIL Score Priority sector lending, types of deposits In India & abroad, Calculation of interest on bank deposits, Modern banking services- Challenges and prospects for modern banking. Corporate banking nature and development in corporate banking Consortium finance, Insolvency and bankruptcy code 2016. Dr.S.Sangeetha, Associate Professor coordinated this visit. 2017 Managerial Economics course Local Companies on August 19, 2017 180 students of batch 2017-19, visited various local companies like Aquasub Engineering, Eastern Spinwell Limited, Gesco, Jayam Engineering, Mas Solar System, Mouli Technologies, Ponmani Wet Grinder, Sairam Polymers, Southern Engineering , Vikashini Spinners Rojas Jute Company, Hifit Engineering Pvt Ltd, Kool Tek Industries , BERO Enterprise and Distributions ltd , Sri Farms , MechLab Equipments, Coimbatore; Cheran Pumps, Coimbatore; SS Engineering, Kariyampalayam, MillTech CNC, Coimbatore; Perfect Tools and Die, Perfect Tools and Dye; in and around Coimbatore on August 19, 2017. The students made a poster presentation on this visit on September 7, 2017. They collected data and explained the production functions, different types of cost incurred by the company. -

Redalyc.Spatial Prediction of Heavy Metal Pollution for Soils in Coimbatore, India Based on Universal Kriging
International Journal of Combinatorial Optimization Problems and Informatics E-ISSN: 2007-1558 [email protected] International Journal of Combinatorial Optimization Problems and Informatics México Gandhimathi, A.; Meenambal, T. Spatial Prediction of Heavy Metal Pollution for Soils in Coimbatore, India based on universal kriging International Journal of Combinatorial Optimization Problems and Informatics, vol. 4, núm. 2, mayo- agosto, 2013, pp. 31-52 International Journal of Combinatorial Optimization Problems and Informatics Morelos, México Available in: http://www.redalyc.org/articulo.oa?id=265229633004 How to cite Complete issue Scientific Information System More information about this article Network of Scientific Journals from Latin America, the Caribbean, Spain and Portugal Journal's homepage in redalyc.org Non-profit academic project, developed under the open access initiative © International Journal of Combinatorial Optimization Problems and Informatics, Vol. 4, No. 2, May-Aug 2013, pp. 31-52. ISSN: 2007-1558. Spatial Prediction of Heavy Metal Pollution for Soils in Coimbatore, India based on universal kriging A.Gandhimathi, Department of Civil Engg, Kumaraguru College of Technology, Dr.T.Meenambal Department of Civil Engg, Government College of Technology, Coimbatore, Tamil Nadu, India. ABSTRACT Coimbatore is a fast growing city, Manchester of Tamil Nadu, India. In Coimbatore Industry effluents and wastes being discharged randomly on soil, river, lake and road side without any treatment. They pollute productive soil, natural water system as well as ground water. Assessment of heavy metal content in soil and wetland from various localities of Coimbatore, Tamil Nadu was undertaken. Heavy metal pollution generally a non-stationary variable, the technique of universal kriging is applied in preference to ordinary kriging as the interpolation method.