A Simple Differentiation Protocol for Generation of Induced Pluripotent Stem Cell-Derived Basal Forebrain-Like Cholinergic Neuro
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Main Hypotheses, Concepts and Theories in the Study of Alzheimer's Disease
An, et al, Main hypotheses, concepts and theories in the study of Alzheimer’s disease Main hypotheses, concepts and theories in the study of Alzheimer’s disease Yuhui An*, Chao Zhang, Siyu He, Chunxia Yao, Limei Zhang, Qian Zhang Department of Biochemistry and Molecular Biology, Basic Medical College, Zhengzhou University, Zhengzhou, Henan 450052, China Received September 2, 2008 Abstract The incidence of Alzheimer’s disease (AD) is 25 millions worldwide in 2000 and it is expected to increase to 63 and 114 millions in 2030 and 2050, respectively. Nowadays, such aging disease has caused enormous medical and financial burden to the community, which effective prevention and treatment are urgently needed. In this study, we have reviewed different hypotheses, concepts and theories of AD. These include hypothesis related to the loss of cholinergic neuron, calcium, oxidative imbalance, microtubule instability and amyloid cascade; the concepts about mild cognitive impair- ment and the regulation and interference of original molecule; and the theories of nitric oxide and glutamate neurotoxic- ity. Although genetic tests have existed for the research of AD, they are considered useful only for the small number of families with a history of early-onset illness. Because AD is a genetically heterogeneous disorder, it is classified as familial and sporadic. We hope this review can briefly provide a summary of the general knowledge about sporadic AD, and help to promote the research on AD or related prevention and treatment. [Life Science Journal. 2008; 5(4): 1 – 5] (ISSN: 1097 – 8135). Keywords: Alzheimer’s disease; cognitive impairment; memory loss; hypothesis; conception; theory 1 Introduction Although genetic tests have existed for the research of Alzheimer disease, they are considered useful only for According to a report of world heath organization the small number of families with a history of early-onset (WHO)[1], the incidence of Alzheimer’s disease (AD), a illness. -

Α7 Nicotinic Receptor Up-Regulation in Cholinergic Basal Forebrain Neurons in Alzheimer Disease
ORIGINAL CONTRIBUTION ␣7 Nicotinic Receptor Up-regulation in Cholinergic Basal Forebrain Neurons in Alzheimer Disease Scott E. Counts, PhD; Bin He, MD; Shaoli Che, MD, PhD; Milos D. Ikonomovic, MD; Steven T. DeKosky, MD; Stephen D. Ginsberg, PhD; Elliott J. Mufson, PhD Background: Dysfunction of basocortical cholinergic pro- Participants: Participants were members of the Rush jection neurons of the nucleus basalis (NB) correlates with Religious Orders Study cohort. cognitive deficits in Alzheimer disease (AD). Nucleus ba- Main Outcome Measures: Real-time quantitative poly- salis neurons receive cholinergic inputs and express nico- merase chain reaction was performed to validate micro- tinic acetylcholine receptors (nAChRs) and muscarinic array findings. AChRs (mAChRs), which may regulate NB neuron activ- ity in AD. Although alterations in these AChRs occur in Results: Cholinergic NB neurons displayed a statisti- the AD cortex, there is little information detailing whether cally significant up-regulation of ␣7 nAChR messenger defects in nAChR and mAChR gene expression occur in RNA expression in subjects with mild to moderate AD cholinergic NB neurons during disease progression. compared with those with NCI and MCI (PϽ.001). No differences were found for other nAChR and mAChR sub- types across the cohort. Expression levels of ␣7 nAChRs Objective: To determine whether nAChR and mAChR were inversely associated with Global Cognitive Score and gene expression is altered in cholinergic NB neurons dur- with Mini-Mental State Examination performance. ing the progression of AD. Conclusions: Up-regulation of ␣7 nAChRs may signal Design: Individual NB neurons from subjects diag- a compensatory response to maintain basocortical cho- nosed ante mortem as having no cognitive impairment linergic activity during AD progression. -

Genetics and Molecular Biology, 43, 4, E20190404 (2020) Copyright © Sociedade Brasileira De Genética
Genetics and Molecular Biology, 43, 4, e20190404 (2020) Copyright © Sociedade Brasileira de Genética. DOI: https://doi.org/10.1590/1678-4685-GMB-2019-0404 Short Communication Human and Medical Genetics Influence of a genetic variant of CHAT gene over the profile of plasma soluble ChAT in Alzheimer disease Patricia Fernanda Rocha-Dias1, Daiane Priscila Simao-Silva2,5, Saritha Suellen Lopes da Silva1, Mauro Roberto Piovezan3, Ricardo Krause M. Souza4, Taher. Darreh-Shori5, Lupe Furtado-Alle1 and Ricardo Lehtonen Rodrigues Souza1 1Universidade Federal do Paraná (UFPR), Centro Politécnico, Programa de Pós-Graduação em Genética, Departamento de Genética, Curitiba, PR, Brazil. 2Instituto de Pesquisa do Câncer (IPEC), Guarapuava, PR, Brazil. 3Universidade Federal do Paraná (UFPR), Departamento de Neurologia, Hospital de Clínicas, Curitiba, PR, Brazil. 4 Instituto de Neurologia de Curitiba (INC), Ambulatório de Distúrbios da Memória e Comportamento, Demência e Outros Transtornos Cognitivos e Comportamentais, Curitiba, PR, Brazil. 5Karolinska Institutet, Care Sciences and Society, Department of Neurobiology, Stockholm, Sweden. Abstract The choline acetyltransferase (ChAT) and vesicular acetylcholine transporter (VAChT) are fundamental to neurophysiological functions of the central cholinergic system. We confirmed and quantified the presence of extracellular ChAT protein in human plasma and also characterized ChAT and VAChT polymorphisms, protein and activity levels in plasma of Alzheimer’s disease patients (AD; N = 112) and in cognitively healthy controls (EC; N = 118). We found no significant differences in plasma levels of ChAT activity and protein between AD and EC groups. Although no differences were observed in plasma ChAT activity and protein concentration among ChEI-treated and untreated AD patients, ChAT activity and protein levels variance in plasma were higher among the rivastigmine- treated group (ChAT protein: p = 0.005; ChAT activity: p = 0.0002). -

A Simple Differentiation Protocol for Generation of Induced
1 Article, Special Issue "Induced Pluripotent Stem Cells in Neurodegenerative Diseases: Application for Therapy and 2 Disease Modeling" 3 A simple differentiation protocol for generation of 4 induced pluripotent stem cell-derived basal forebrain 5 cholinergic neurons for Alzheimer’s disease and 6 frontotemporal dementia disease modeling 7 Supplemental information 8 Method 1. Reprogramming and characterisation of MBE2960 healthy control iPSC line 9 The iPSCs were generated using skin fibroblasts obtained from subjects over the age of 18 years 10 by episomal method as described [40]. Briefly, reprogramming was performed on passage 8-10 11 fibroblasts by nucleofection (Lonza Amaxa Nucleofector) with episomal vectors expressing 12 OCT4, SOX2, KLF4, L-MYC, LIN28 and shRNA against p53 [41] in feeder- and serum- free 13 conditions using TeSR-E7 medium (Stemcell Technologies). Subsequently, reprogrammed 14 colonies were manually dissected to establish clonal cell lines [42]. Three clones were assessed 15 for pluripotency markers via immunocytochemistry (Figure S1A). The iPSC line was expanded 16 and characterised. Embryoid bodies were obtained as described [43] and using tri-lineage 17 differentiation kit (Stemcell Technologies). Germ layer differentiation was assessed by 18 immunochemistry (Figure S1B). Copy number variation (CNV) analysis of original fibroblasts 19 and iPSCs from MBE2960 (Figure S1C) was performed using Illumina HumanCore Beadchip 20 arrays as we described [40]. CNV analyses were performed using PennCNV and QuantiSNP with 21 default parameter settings [44,45]. Chromosomal aberrations were deemed to involve at least 10 22 contiguous single nucleotide polymorphisms (SNPs) or a genomic region spanning at least 1MB 23 [44,45]. The B allele frequency (BAF) and the log R ratio (LRR) were extracted from 24 GenomeStudio (Illumina) for representation (Figure S1D). -

Diagnosis of FOXG1 Syndrome Caused by Recurrent Balanced Chromosomal Rearrangements: Case Study and Literature Review Connor P
Craig et al. Mol Cytogenet (2020) 13:40 https://doi.org/10.1186/s13039-020-00506-1 CASE REPORT Open Access Diagnosis of FOXG1 syndrome caused by recurrent balanced chromosomal rearrangements: case study and literature review Connor P. Craig1,2, Emily Calamaro3, Chin‑To Fong3, Anwar M. Iqbal1, Alexander R. Paciorkowski3,4,5,6 and Bin Zhang1,3* Abstract Background: The FOXG1 gene plays a vital role in mammalian brain diferentiation and development. Intra‑ and intergenic mutations resulting in loss of function or altered expression of the FOXG1 gene cause FOXG1 syndrome. The hallmarks of this syndrome are severe developmental delay with absent verbal language, post‑natal growth restric‑ tion, post‑natal microcephaly, and a recognizable movement disorder characterized by chorea and dystonia. Case presentation: Here we describe a case of a 7‑year‑old male patient found to have a de novo balanced translo‑ cation between chromosome 3 at band 3q14.1 and chromosome 14 at band 14q12 via G‑banding chromosome and Fluorescence In Situ Hybridization (FISH) analyses. This rearrangement disrupts the proximity of FOXG1 to a previously described smallest region of deletion overlap (SRO), likely resulting in haploinsufciency. Conclusions: This case adds to the growing body of literature implicating chromosomal structural variants in the manifestation of this disorder and highlights the vital role of cis‑acting regulatory elements in the normal expression of this gene. Finally, we propose a protocol for refex FISH analysis to improve diagnostic efciency for patients with suspected FOXG1 syndrome. Keywords: FOXG1, Haploinsufciency, Postnatal microcephaly, FISH, Enhancer, Chromosomal rearrangement, Diagnosis Introduction brain development, with high levels of expression in the Te Forkhead Box G1 (FOXG1) gene [OMIM: 164874], developing fetal telencephalon [1–4]. -
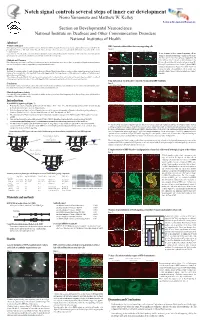
Notch Signal Controls Several Steps of Inner Ear Development Norio Yamamoto and Matthew W
NIDCD Notch signal controls several steps of inner ear development Norio Yamamoto and Matthew W. Kelley Section on Developmental Neuroscience Section on Developmental Neuroscience National Institute on Deafness and Other Communication Disorders National Institutes of Health Abstract Problem addressed RBP-J mutant cochleae did not have any supporting cells Notch signaling has been reported to contribute to inner ear development, however, its specific functions remain unclear, partly because of discrepancies between the phenotypes of mutant mice with single deletion of specific Notch related genes. These discrepancies are probably due to functional compensation by other Notch Figure 4 receptors or ligands. Foxg1 Cre;RBP-J floxed/+ Foxg1 Cre;RBP-J floxed/floxed A B C D To determine the effects of complete elimination of Notch signaling, we used a conditional knockout of the Rbpsuh gene. RBP-J protein is a critical transcriptional p27 Phalloidin p27 Phalloidin To test if mutant cochleae contained supporting cells we co-activator for all Notch molecules and thus deletion of this protein inhibits all Notch signaling. examined expression of supporting cell markers such as p27 and Prox1. On E17.5 p27 was expressed in supporting cells Methods and Measures ***** ***** under or between hair cells such as inner pharyngeal cells, Floxed Rbpsuh mice were crossed with Foxg1-Cre knock-in mice to delete the Rbpsuh gene in the inner ear. Inner ear phenotypes in Rbpsuh conditional knockout Deiter's cells and pillar cells (asterisks in figure 4 A and B). mice were determined at various developmental stages using immunohistochemistry. But no p27 expression was detected in those regions of RBP-J E Prox1 F Phalloidin G Prox1 H Phalloidin conditional knockout mice (Figure 4 C and D). -

Acetylcholine Revisited Were Effective Does Suggest That the Observed Effect Was Due to Ach Release Anders Bjorklund and Stephen B
NEWS AND VIEWS COGNITIVE FUNCTION------------------------------ bearing the choline acetyltransferase gene Acetylcholine revisited were effective does suggest that the observed effect was due to ACh release Anders Bjorklund and Stephen B. Dunnett and activation of cholinergic receptors in the area surrounding the transplants. This IF impaired cortical cholinergic function afferent neuronal connections. is compatible with studies in which deficits associated with damage to the basal fore This brings us to what the new studl associated with forebrain cholinergic brain cholinergic neuron system is in can tell us about the normal role of the damage are alleviated by cholinomimetic strumental in dementia-related cognitive forebrain cholinergic system in cognitive drugs such as physostigmine or tacrine. declinel,2, then restoration of forebrain function. Interpretation of the data of Moreover, non-cholinergic drugs that act cholinergic neurotransmission might be Winkler et al. is by no means straightfor to enhance cortical function in a more enough to improve at least some aspects of ward in this respect. There is the question general way can provide a similar recovery impaired learning and memory, particu of specificity at three levels- whether the of the behavioural deficits associated with larly in conditions such as Alzheimer's functional deficit induced by excitotoxic NBM lesions and other types of cho 11 12 disease. The neurotransmitter acetylcho NBM lesions (and, by inference, in linergic blockade • . This suggests that a line (ACh) is suspected to be an important Alzheimer's dementia) is indeed cho pharmacological reversal of deficits acting participant in the maintenance of normal linergic, cortical and cognitive in nature, at the neocortical level can be mediated cognitive function, but it is not clear to which may not be the case. -

Downloaded from Bioscientifica.Com at 09/26/2021 08:38:14PM Via Free Access
1 185 L C Gregory, P Gergics, OTX2 mutations in congenital 185:1 121–135 Clinical Study M Nakaguma and others hypopituitarism The phenotypic spectrum associated with OTX2 mutations in humans Louise C Gregory 1,*, Peter Gergics2,* , Marilena Nakaguma3,* , Hironori Bando2 , Giuseppa Patti1,4,5, Mark J McCabe1, Qing Fang 2, Qianyi Ma2 , Ayse Bilge Ozel2 , Jun Z Li2 , Michele Moreira Poina3, Alexander A L Jorge 3, Anna F Figueredo Benedetti3, Antonio M Lerario3, Ivo J P Arnhold3 , Berenice B Mendonca3 , Mohamad Maghnie4,5, Sally A Camper2 , Luciani R S Carvalho3 and Mehul T Dattani1 1Section of Molecular Basis of Rare Disease, Genetics and Genomic Medicine Research & Teaching Department, UCL Great Ormond Street Institute of Child Health, London, UK, 2Department of Human Genetics, University of Michigan, Correspondence Ann Arbor, Michigan, USA, 3Developmental Endocrinology Unit, Hospital das Clinicas da Faculdade de Medicina da should be addressed Universidade de São Paulo, São Paulo, Brazil, 4Department of Pediatrics, IRCCS Istituto Giannina Gaslini and to S A Camper or M T 5Department of Neuroscience, Rehabilitation, Ophthalmology, Genetics, Maternal and Child Health, University of Dattani Genova, Genova, Italy Email *(L C Gregory, P Gergics and M Nakaguma contributed equally to this work) [email protected] or [email protected] Abstract Objective: The transcription factor OTX2 is implicated in ocular, craniofacial, and pituitary development. Design: We aimed to establish the contribution of OTX2 mutations in congenital hypopituitarism patients with/without eye abnormalities, study functional consequences, and establish OTX2 expression in the human brain, with a view to investigate the mechanism of action. Methods: We screened patients from the UK (n = 103), international centres (n = 24), and Brazil (n = 282); 145 were within the septo-optic dysplasia spectrum, and 264 had no eye phenotype. -
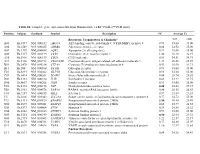
Table S1. Complete Gene Expression Data from Human Diabetes RT² Profiler™ PCR Array Receptors, Transporters & Channels* A
Table S1. Complete gene expression data from Human Diabetes RT² Profiler™ PCR Array Position Unigene GenBank Symbol Description FC Average Ct Receptors, Transporters & Channels* NGT GDM A01 Hs,5447 NM_000352 ABCC8 ATP-binding cassette, sub-family C (CFTR/MRP), member 8 0.93 35.00 35.00 A04 0Hs,2549 NM_000025 ADRB3 Adrenergic, beta-3-, receptor 0.88 34.92 35.00 A07 Hs,1307 NM_000486 AQP2 Aquaporin 2 (collecting duct) 0.93 35.00 35.00 A09 30Hs,5117 NM_001123 CCR2 Chemokine (C-C motif) receptor 2 1.00 26.28 26.17 A10 94Hs,5916 396NM_006139 CD28 CD28 molecule 0.81 34.51 34.71 A11 29Hs,5126 NM_001712 CEACAM1 Carcinoembryonic antigen-related cell adhesion molecule 1 1.31 26.08 25.59 B01 82Hs,2478 NM_005214 CTLA4 (biliaryCytotoxic glycoprotein) T-lymphocyte -associated protein 4 0.53 30.90 31.71 B11 24Hs,208 NM_000160 GCGR Glucagon receptor 0.93 35.00 35.00 C01 Hs,3891 NM_002062 GLP1R Glucagon-like peptide 1 receptor 0.93 35.00 35.00 C07 03Hs,6434 NM_000201 ICAM1 Intercellular adhesion molecule 1 0.84 28.74 28.89 D02 47Hs,5134 NM_000418 IL4R Interleukin 4 receptor 0.64 34.22 34.75 D06 57Hs,4657 NM_000208 INSR Insulin receptor 0.93 35.00 35.00 E05 44Hs,4312 NM_006178 NSF N-ethylmaleimide-sensitive factor 0.48 28.42 29.37 F08 79Hs,2961 NM_004578 RAB4A RAB4A, member RAS oncogene family 0.88 20.55 20.63 F10 69Hs,7287 NM_000655 SELL Selectin L 0.97 23.89 23.83 F11 56Hs,3806 NM_001042 SLC2A4 Solute carrier family 2 (facilitated glucose transporter), member 4 0.77 34.72 35.00 F12 91Hs,5111 NM_003825 SNAP23 Synaptosomal-associated protein, 23kDa 3.90 -

14Q12q13.3 Deletion Diagnosed Using Chromo Somal Microarray
Case Report Neonatal Med 2020 November;27(4):207-213 https://doi.org/10.5385/nm.2020.27.4.207 pISSN 2287-9412 . eISSN 2287-9803 14q12q13.3 Deletion Diagnosed Using Chromo somal Microarray Analysis in an Infant Showing Seizures, Hypoplasia of the Corpus Callosum, and Developmental Delay Jae Hyuk Kwon, MD1, Young Hwa Song, MD1, Jung Min Yoon, MD1, Eun Jung Cheon, MD1, Kyung Ok Ko, MD1, Jae Woo Lim, MD1, and Hyon J. Kim, MD, FACMG2 Departments of 1Pediatrics and 2Medical Genetics, Konyang University College of Medicine, Daejeon, Korea ABSTRACT Received: 18 August 2020 Revised: 25 September 2020 14q12q13.3 Deletion is a rare microdeletion syndrome associated with neurodevelop Accepted: 6 October 2020 mental delay, failure to thrive, seizures, and abnormal brain development. Symptoms Correspondence to: Hyon J. Kim, MD vary depending on the sites of gene deletion, and establishing the diagnosis is often Department of Medical Genetics, difficult, as the condition cannot be detected with routine chromosome analysis. In Konyang University College of Medi this report, we present a patient with intrauterine growth retardation, microcephaly, cine, 158 Gwanjeodongro, Seogu, muscle weakness, seizures, and hypoplasia of the corpus callosum who underwent Daejeon 35365, Korea diagnostic tests, including karyotyping in the neonatal period without leading to a Tel: +82426009230 specific diagnosis. The patient was confirmed with a serious developmental disorder, Fax: +82426009090 and a chromosomal microarray analysis was performed at 8 months of age, revealing Email: [email protected] a 14q12q13.3 deletion. In this case, the condition was diagnosed in early infancy, in contrast to previously reported cases, and the patient had diverse and severe symptoms. -

The Relationship Between Cholinergic and Noradrenergic Activity and Behavioral State
THE RELATIONSHIP BETWEEN CHOLINERGIC AND NORADRENERGIC ACTIVITY AND BEHAVIORAL STATE by JOHN JOSEPH FRANCIS A THESIS Presented to the Department of Biology and the Robert D. Clark Honors College in partial fulfillment of the requirements for the degree of Bachelor of Science May 2021 An Abstract of the Thesis of John Joseph Francis for the degree of Bachelor of Science in the Department of Biology to be taken June 2021 Title: The Relationship Between Cholinergic and Noradrenergic Activity and Behavioral State Approved: ______David McCormick, Ph.D.____ Primary Thesis Advisor Approved: ______Lindsay Collins, Ph.D.________ Second Reader Approved: ___ Adam Miller, Ph.D._ ______ Biology Honors Faculty Representative Approved: _______Chris Sinclair, Ph.D._______ Clark Honors College Representative Animal behaviors result from complex network activity in the brain. Precise excitation and inhibition within these networks are partially regulated by neuromodulatory systems that regulate the behavior of other neurons, influencing brain processing and ultimately the animal’s behavior. This regulation is accomplished, in part, by the neuromodulators acetylcholine (ACh) and noradrenaline (NA). ACh and NA are produced and released by cholinergic and noradrenergic neurons, respectively, and have broad functions throughout the central nervous system. This project investigates the relationship between ACh and NA neuromodulatory activity and ii behavioral state with respect to arousal and behavior-dependent modes of neuromodulation. Using systems neuroscience techniques, such as intracranial viral injections and two-photon microscopy, this project offers novel insights into the dynamic relationship between ACh and NA activity and behavioral state in mice. First, I confirm previous findings of a strong relationship between neuromodulatory activity and arousal state, as measured by walking velocity, whisking, and pupil dilation/constriction. -

Grimme, Acadia.Pdf
MECHANISM OF ACTION OF HISTONE DEACETYLASE INHIBITORS ON SURVIVAL MOTOR NEURON 2 PROMOTER by Acadia L. Grimme A thesis submitted to the Faculty of the University of Delaware in partial fulfillment of the requirements for the degree of Bachelors of Science in Biological Sciences with Distinction Spring 2018 © 2018 Acadia Grimme All Rights Reserved MECHANISM OF ACTION OF HISTONE DEACETYLASE INHIBITORS ON SURVIVAL MOTOR NEURON 2 PROMOTER by Acadia L. Grimme Approved: __________________________________________________________ Matthew E. R. Butchbach, Ph.D. Professor in charge of thesis on behalf of the Advisory Committee Approved: __________________________________________________________ Deni S. Galileo, Ph.D. Professor in charge of thesis on behalf of the Advisory Committee Approved: __________________________________________________________ Carlton R. Cooper, Ph.D. Committee member from the Department of Biological Sciences Approved: __________________________________________________________ Gary H. Laverty, Ph.D. Committee member from the Board of Senior Thesis Readers Approved: __________________________________________________________ Michael Chajes, Ph.D. Chair of the University Committee on Student and Faculty Honors ACKNOWLEDGMENTS I would like to acknowledge my thesis director Dr. Butchbach for his wonderful guidance and patience as I worked through my project. He has been an excellent research mentor over the last two years and I am forever thankful that he welcomed me into his lab. His dedication to his work inspires me as an aspiring research scientist. His lessons will carry on with me as I pursue future research in graduate school and beyond. I would like to thank both current and former members of the Motor Neuron Disease Laboratory: Sambee Kanda, Kyle Hinkle, and Andrew Connell. Sambee and Andrew patiently taught me many of the techniques I utilized in my project, and without them it would not be what it is today.