OGBIYE ADEBANJI SAMUEL B.Sc (Hons); M.Sc, MNSE, COREN REGD ENGR (CUO3GP0030)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Annual Report of the Colonies. Nigeria 1898
This document was created by the Digital Content Creation Unit University of Illinois at Urbana-Champaign 2010 COLONIAL REPORTS—ANNUAL. No. 284. LAGOS, REPORT FOR 1898. (For Report for 1897, see No. 832.) tyimnti* to totf) tyQum of ^aiclianunt b# OIomman& of pjt fflawtfr December, 1899. 1 LONDON. PRINTED FOE HER MAJESTTS STATIONERY OFPIOB, BY DARLING & SON, LTD., 1-3, GREAT ST THOMAS APOSTLE, 1S.O. And to be purchased, either directly w th- %ny Bookseller, from E'iiiE & SPOTTISWOODE. EAS" TARI>IN'.J- SI: J. FLEET 8?ss*-r, AJ.O. tad 82, ABINODOH STREET, "WEd-*v;n,3I:J.8T S.W.; or JOHN MENZIES & Co., 12, HANOVER STREET, EDINB&BOH, tad DO, WEST ±!ILE STREET, GLASGOW ; or HODGES, FIGGIS, & Co., LIMIT J>, 104, GBJUTTOX grant, A>TOU* 1890. COLONIAL REPORTS. The following, among other, reports relating to Her Majesty's Colonial Possessions have been issued, and may be obtained for a few pence from the sources indicated on the title page :— ANNUAL. No. Colony. Year. 255 Basutoland • • • • • • • • • 1897-98 256 Newfoundland - •»• 1896-97 257 Cocos-Keeling and Christmas Islands 1898 258 British New Guinea ... •» • * • • • • • 1897-98 259 Bermuda • * * • • e • • • 1898 260 Niger.—West African Frontier \ Force 1897-98 261 Jamaica ... • • • ft 262 Barbados .. • • • • • • • • • 1898 263 Falkland Islands • • • • *« * • • * )>t 264 Gambia ... • • • • • • • • • f* 265 St. Helena • • • • • • * • • ft 266 Leeward Islands ... • • • ft 267 St. Lucia... • • • ••• 1* 268 JEP&j x • • • • • • * *» • • • • •«• »•# t'l 269 Tarks and Caicos Islands • * • ••* ••• ft 270 Al£vlt& ••• • • <* • 6-0. • • • ft 271 Gold Coast • • • •0* ft 272 Trinidad • • • «*• • *f 273 Sierra Leone • • • 0 * • • * • ft 274 Ceylon ... • • • ff • • • • • ft 275 British Solomon Islands • • • « ft » • • • 1898-99 276 Gibraltar f • * 1898 277 Bahamas tf 278 British Honduras ...... -
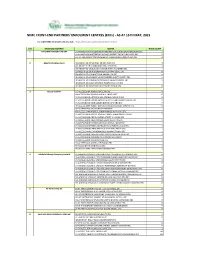
NIMC FRONT-END PARTNERS' ENROLMENT CENTRES (Ercs) - AS at 15TH MAY, 2021
NIMC FRONT-END PARTNERS' ENROLMENT CENTRES (ERCs) - AS AT 15TH MAY, 2021 For other NIMC enrolment centres, visit: https://nimc.gov.ng/nimc-enrolment-centres/ S/N FRONTEND PARTNER CENTER NODE COUNT 1 AA & MM MASTER FLAG ENT LA-AA AND MM MATSERFLAG AGBABIAKA STR ILOGBO EREMI BADAGRY ERC 1 LA-AA AND MM MATSERFLAG AGUMO MARKET OKOAFO BADAGRY ERC 0 OG-AA AND MM MATSERFLAG BAALE COMPOUND KOFEDOTI LGA ERC 0 2 Abuchi Ed.Ogbuju & Co AB-ABUCHI-ED ST MICHAEL RD ABA ABIA ERC 2 AN-ABUCHI-ED BUILDING MATERIAL OGIDI ERC 2 AN-ABUCHI-ED OGBUJU ZIK AVENUE AWKA ANAMBRA ERC 1 EB-ABUCHI-ED ENUGU BABAKALIKI EXP WAY ISIEKE ERC 0 EN-ABUCHI-ED UDUMA TOWN ANINRI LGA ERC 0 IM-ABUCHI-ED MBAKWE SQUARE ISIOKPO IDEATO NORTH ERC 1 IM-ABUCHI-ED UGBA AFOR OBOHIA RD AHIAZU MBAISE ERC 1 IM-ABUCHI-ED UGBA AMAIFEKE TOWN ORLU LGA ERC 1 IM-ABUCHI-ED UMUNEKE NGOR NGOR OKPALA ERC 0 3 Access Bank Plc DT-ACCESS BANK WARRI SAPELE RD ERC 0 EN-ACCESS BANK GARDEN AVENUE ENUGU ERC 0 FC-ACCESS BANK ADETOKUNBO ADEMOLA WUSE II ERC 0 FC-ACCESS BANK LADOKE AKINTOLA BOULEVARD GARKI II ABUJA ERC 1 FC-ACCESS BANK MOHAMMED BUHARI WAY CBD ERC 0 IM-ACCESS BANK WAAST AVENUE IKENEGBU LAYOUT OWERRI ERC 0 KD-ACCESS BANK KACHIA RD KADUNA ERC 1 KN-ACCESS BANK MURTALA MOHAMMED WAY KANO ERC 1 LA-ACCESS BANK ACCESS TOWERS PRINCE ALABA ONIRU STR ERC 1 LA-ACCESS BANK ADEOLA ODEKU STREET VI LAGOS ERC 1 LA-ACCESS BANK ADETOKUNBO ADEMOLA STR VI ERC 1 LA-ACCESS BANK IKOTUN JUNCTION IKOTUN LAGOS ERC 1 LA-ACCESS BANK ITIRE LAWANSON RD SURULERE LAGOS ERC 1 LA-ACCESS BANK LAGOS ABEOKUTA EXP WAY AGEGE ERC 1 LA-ACCESS -

South – West Zone
South – West Zone Ogun State Contact Number/Enquires ‐08033251216 S/N City / Town Street Address 1 Abigi Abisi Main Garage 2 Aiyepe Ikenne Local Government Secretariat, Ikenne 1 3 Aiyepe Ikenne Local Government Secretariat, Ikenne 2 4 Aiyepe Ikenne Ilisan Palace 5 Aiyetoro Ayetoro Palace 6 Ake Itoku Market 7 Ake Ake Palace 8 Ake Osile Palace 9 Ake Olumo Tourist Center 10 Atan Ijebu Igbo (Abusi College) 11 Atan Ago Iwoye (Ebumawe Palace) 12 Atan Atan Local Government Secretariat 13 Atan Alasa Market 14 Atan Oba’s Palace 15 Atan Alaga Market 16 Ewekoro Itori, Near Local Government Secretariat 1 17 Ewekoro Itori, Near Local Government Secretariat 2 18 Ifo Ogs Plaza, Ajuwon 19 Ifo Ijoko Last Bus Stop 20 Ifo Akute Market 21 Ifo Ifo Market 22 Ifo Agbado, Rail Crossing 23 Ifo Agbado/Opeilu, Junction 1 24 Ifo Agbado/Opeilu, Junction 2 25 Ijebu Igbo Oru Garage, Oru 1 26 Ijebu Igbo Station 27 Sagamu Portland Cement Gate 28 Sagamu Moresimi 29 Sagamu NNPC Gate 30 Ota Covenant University Gate 31 Ota Covenant Central Auditorium 32 Ota Covenant University Female Hostel 1 33 Ota Covenant University Male Hostel 1 34 Redeem Camp Redeemers University Gate 35 Redeem Camp Redeemers University Admin Office 36 Redeem Camp Main Gate 37 Ogere Old Toll Gate (Lagos Side) 38 Ogere Old Toll Gate (Ibadan Side) 39 UNAAB University Of Agriculture Gate 40 UNAAB UNAAB Student Building 41 Odogbolu Government College, Odogbolu 42 Osu Ogun State University Gate 43 Osu Ogun State University Main Campus 44 Ijebu Igbo Oru Garage, Oru 2 45 Ilaro Ilaro, Sayedero 46 Ilaro Orita -

Joint Admissions and Matriculation Board
JOINT ADMISSIONS AND MATRICULATION BOARD APPLICATION STATISTICS BY INTITUTION AND GENDER (AGE LESS THAN 16) S/NO INSTITUTION F M TOTAL 1 ABUBAKAR TAFAWA BALEWA UNIVERSITY, BAUCHI, BAUCHI STATE 78 89 167 2 ACHIEVERS UNIVERSITY, OWO, ONDO STATE 3 0 3 3 ADAMAWA STATE UNIVERSITY, MUBI, ADAMAWA STATE 8 5 13 4 ADEKUNLE AJASIN UNIVERSITY, AKUNGBA-AKOKO, ONDO STATE 169 68 237 5 ADELEKE UNIVERSITY, EDE, OSUN STATE 6 4 10 6 ADEYEMI COLLEGE OF EDUCATION, ONDO STATE. (AFFL TO OAU, ILE-IFE) 8 4 12 7 ADEYEMI COLLEGE OF EDUCATION, ONDO, ONDO STATE 1 0 1 8 AFE BABALOLA UNIVERSITY, ADO-EKITI, EKITI STATE 92 71 163 9 AHMADU BELLO UNIVERSITY TEACHING HOSPITAL, ZARIA, KADUNA STATE 2 0 2 10 AHMADU BELLO UNIVERSITY, ZARIA, KADUNA STATE 826 483 1309 11 AIR FORCE INSTITUTE OF TECHNOLOGY, KADUNA, KADUNA STATE 2 1 3 12 AJAYI CROWTHER UNIVERSITY, OYO, OYO STATE 6 1 7 13 AKANU IBIAM FEDERAL POLYTECHNIC, UNWANA, AFIKPO, EBONYI STATE 5 3 8 14 AKWA IBOM STATE UNIVERSITY, IKOT-AKPADEN, AKWA IBOM STATE 39 28 67 15 AKWA-IBOM STATE POLYTECHNIC, IKOT-OSURUA, AKWA IBOM STATE 7 3 10 16 ALEX EKWUEME FEDERAL UNIVERSITY, NDUFU-ALIKE, EBONYI STATE 55 33 88 17 AL-HIKMAH UNIVERSITY, ILORIN, KWARA STATE 3 1 4 18 AL-QALAM UNIVERSITY, KATSINA, KATSINA STATE 6 1 7 19 ALVAN IKOKU COLLEGE OF EDUCATION, IMO STATE, (AFFL TO UNIV OF NIGERA, NSUKKA) 3 1 4 20 AMBROSE ALLI UNIVERSITY, EKPOMA, EDO STATE 208 117 325 21 AMERICAN UNIVERSITY OF NIGERIA, YOLA, ADAMAWA STATE 4 8 12 22 AMINU DABO COLLEGE OF HEALTH SCIENCE AND TECHNOLOGY, KANO, KANO STATE 1 0 1 23 ANCHOR UNIVERSITY, AYOBO, LAGOS STATE -

MICROFINANCE BANKS (Mfbs) GRANTED PROVISIONAL APPROVAL for NEW LICENCE
MICROFINANCE BANKS (MFBs) GRANTED PROVISIONAL APPROVAL FOR NEW LICENCE S/N MFB STATE 1 ADAIGBO MFB DELTA 2 AKUDIEWA MFB ENUGU 3 AKWENGWU MFB KOGI 4 ALTITUDE MFB LAGOS 5 BABURA MFB EKITI 6 BARNAWA MFB KADUNA 7 BROADVIEW MFB LAGOS 8 CHIBUEZE MFB ABIA 9 CKC MFB RIVERS 10 COMPLETE TRUST MFB LAGOS 11 CREDIT EXPRESS MFB LAGOS 12 CROWNED EAGLE MFB LAGOS 13 E-BARCLAYS MFB ABUJA 14 EDS MFB LAGOS 15 ENTERPRISE MFB LAGOS 16 EQUATOR MFB BAYELSA 17 EWT MFB ABUJA 18 EXPRESS MFB ABIA 19 FIELDREAMS MFB LAGOS 20 FIRST OMASI MFB ENUGU 21 FUTO MFB IMO 22 GARDEN CITY MFB RIVERS 23 GOLDEN CHOICE MFB RIVERS 24 GTI MFB LAGOS 25 I.C GLOBAL MFB DELTA 26 IGANGAN MFB OYO 27 IHECHIOWA MFB ABIA 28 IKORODU DIVISION MFB LAGOS 29 IMSU MFB IMO 30 ISHIAGU MFB ANAMBRA 31 ISUOFIA MFB ANAMBRA 32 IVIE MFB EDO 33 JHN MFB ABUJA 34 KARIS MFB OGUN 35 KOGI MFB KOGI 36 KSF MFB LAGOS 37 LANDGOLD MFB OGUN 38 LEGEND MFB OSUN 39 LEKKI MFB LAGOS 40 LOFTY HEIGHTS MFB EDO 41 MAXITRUST MFB RIVERS 42 NDIOLU MFB ANAMBRA 43 NEIGHBOURHOOD MFB RIVERS 44 NEW-LIFE MFB LAGOS 45 NKPOLU-UST MFB RIVERS 46 OCON SUCCESS MFB OGUN 1 47 ODU MFB KOGI 48 OLIVE MFB LAGOS 49 OSINA MFB IMO 50 OSOMHE MFB EDO 51 PARALLEX MFB LAGOS 52 PROSPERITY MFB EDO 53 REALITY MFB OYO 54 RIGGS MFB LAGOS 55 TOKI RAINBOW MFB RIVERS 56 TRADERS MFB LAGOS 57 ULTIMATE MFB LAGOS 58 AMAIFEKE MFB IMO 59 BERACHAH MFB LAGOS 60 CEDEP MFB BAUCHI 61 COASTLINE MFB DELTA 62 CONFLUENCE MFB KOGI 63 IHIMA MFB KOGI 64 NEWAGE MFB ONDO 65 ORIE-ORBA MFB ENUGU 66 ABIGI MFB OGUN 67 ABIRIBA MFB ABIA 68 AGULERI MFB ANAMBRA 69 AKESAN MFB OYO CR. -

Distribution of Naturally Occuring Radionuclides and Mineralogical Characterization of Ijebu-Ife, Ogun State Nigeria
DISTRIBUTION OF NATURALLY OCCURING RADIONUCLIDES AND MINERALOGICAL CHARACTERIZATION OF IJEBU-IFE, OGUN STATE NIGERIA. BY OLAGBAJU PETER OLUWADAMILARE INTRODUCTION ➢Natural radionuclide's and Environment (UNSCEAR, 2000). ➢Variation of natural radionuclides ➢Sources of natural radionuclides GEOGRAPHY AND GEOLOGY OF STUDY AREA ❖Ijebu-Ife is situated at 604610011 to 604810011 North latitude, 400013011 to 400310011 East longitude. With 67 meters elevation above sea level. ❖Ijebu-Ife, lies within the Dahomey basin which is an inland, offshore, coastal sedimentary basin in the Gulf of Guinea. ❖Sand stone and Lime stone cover almost the whole portion of the study area. IJEBU-IFEAND ENVIRONS 3055’00” 3057’00” 0 4001’00” 4003’00” 0 6 3 59’00” 4 05’00”0 Isonyin 50’00” 50’00” 0 6 6 0 48’00” Ogun-state 48’00” 0 6 Ijebu-Imushin TAI SOLARIN UNIVERSITY Ijebu-Ife 6 0 46’00” OGUN STATE Ijebu-Itele Ijebu-Ife 46’00” 0 6 LEGEND 6 0 44’00” Alluvium Sands and clay 44’00” 0 Sands, clays and shale 6 Odo lewu Igara Shale and limestone Sandstone and limestone 6 0 42’00” Porphyritic granite Geological boundary 42’00” 0 6 Dual carriage Main roads 6 0 Minor roads 40’00” Main paths 40’00” 0 3055’00”6 3057’00” 3059’00” 4001’00” 4003’00” 4005’00” 0 2.0 4.0km JUSTIFICATION UNSCEAR, (1993) Soil as a direct source (IAEA, 1990) Knowledge of background radiation level AIM AND OBEJECTIVES The general objective of this study is to determine the naturally occurring radionuclides and mineralogical characterization of Ijebu-Ife, Ogun State, Nigeria. -

Odo/Ota Local Government Secretariat, Sango - Agric
S/NO PLACEMENT DEPARTMENT ADO - ODO/OTA LOCAL GOVERNMENT SECRETARIAT, SANGO - AGRIC. & BIO. ENGINEERING 1 OTA, OGUN STATE AGEGE LOCAL GOVERNMENT, BALOGUN STREET, MATERNITY, AGRIC. & BIO. ENGINEERING 2 SANGO, AGEGE, LAGOS STATE AHMAD AL-IMAM NIG. LTD., NO 27, ZULU GAMBARI RD., ILORIN AGRIC. & BIO. ENGINEERING 3 4 AKTEM TECHNOLOGY, ILORIN, KWARA STATE AGRIC. & BIO. ENGINEERING 5 ALLAMIT NIG. LTD., IBADAN, OYO STATE AGRIC. & BIO. ENGINEERING 6 AMOULA VENTURES LTD., IKEJA, LAGOS STATE AGRIC. & BIO. ENGINEERING CALVERTON HELICOPTERS, 2, PRINCE KAYODE, AKINGBADE MECHANICAL ENGINEERING 7 CLOSE, VICTORIA ISLAND, LAGOS STATE CHI-FARM LTD., KM 20, IBADAN/LAGOS EXPRESSWAY, AJANLA, AGRIC. & BIO. ENGINEERING 8 IBADAN, OYO STATE CHINA CIVIL ENGINEERING CONSTRUCTION CORPORATION (CCECC), KM 3, ABEOKUTA/LAGOS EXPRESSWAY, OLOMO - ORE, AGRIC. & BIO. ENGINEERING 9 OGUN STATE COCOA RESEARCH INSTITUTE OF NIGERIA (CRIN), KM 14, IJEBU AGRIC. & BIO. ENGINEERING 10 ODE ROAD, IDI - AYANRE, IBADAN, OYO STATE COKER AGUDA LOCAL COUNCIL, 19/29, THOMAS ANIMASAUN AGRIC. & BIO. ENGINEERING 11 STREET, AGUDA, SURULERE, LAGOS STATE CYBERSPACE NETWORK LTD.,33 SAKA TIINUBU STREET. AGRIC. & BIO. ENGINEERING 12 VICTORIA ISLAND, LAGOS STATE DE KOOLAR NIGERIA LTD.,PLOT 14, HAKEEM BALOGUN STREET, AGRIC. & BIO. ENGINEERING OPP. TECHNICAL COLLEGE, AGIDINGBI, IKEJA, LAGOS STATE 13 DEPARTMENT OF PETROLEUM RESOURCES, 11, NUPE ROAD, OFF AGRIC. & BIO. ENGINEERING 14 AHMAN PATEGI ROAD, G.R.A, ILORIN, KWARA STATE DOLIGERIA BIOSYSTEMS NIGERIA LTD, 1, AFFAN COMPLEX, 1, AGRIC. & BIO. ENGINEERING 15 OLD JEBBA ROAD, ILORIN, KWARA STATE Page 1 SIWES PLACEMENT COMPANIES & ADDRESSES.xlsx S/NO PLACEMENT DEPARTMENT ESFOOS STEEL CONSTRUCTION COMPANY, OPP. SDP, OLD IFE AGRIC. & BIO. ENGINEERING 16 ROAD, AKINFENWA, EGBEDA, IBADAN, OYO STATE 17 FABIS FARMS NIGERIA LTD., ILORIN, KWARA STATE AGRIC. -

Ogun State Water Corporation Investment Plan
OGUN STATE WATER CORPORATION INVESTMENT PLAN PRESIDENTIAL BOULEVARD, OKE-MOSAN, ABEOKUTA, NIGERIA Website: www.ogunwater.org.ng MAY 2010 1 / OGUN STATE WATER PROJECTS STATE, FEDERAL AND MULTILATERAL SUBMISSION TO THE HONOURABLE MINISTER, FEDERAL MINISTRY OF WATER RESOURCES REF. NATIONAL COUNCIL MEETING OF 17 TH TO 18 TH MAY 2010 Website: www.ogunwater.org.ng 2 / OGUN STATE WATER CORPORATION BILL OF QUANTITIES OF PROPOSED URBAN PROJECTS 1. Rehabilitation Works for Ikangba Housing Estate 2. Rehabilitation Works for Ogere, Isara, Ipara, Ikenne and Ode Remo 3. Laying of 4km 300mmD.I pipe secondary mains from Asero to Osiele to Odeda, 4. Relocation of Abeokuta Old Scheme Rising Main to Iberekodo Reservior with 300mm Ductile Iron PIPE Distance 2KM 5. 150mmØ Distribution Pipeline to Mawuko-Fehure 6. Relocation and Replacement of 300mm Ø Asbestos Cement Pipe with 200mm HDPE Pipe from Fehure to University of Agriculture Alabata Dist 18km 7. Pipeline Extension to Bode Olude - 5 kilometres in 200mmØ HDPE 8. Panseke Booster Station completion 9. Idi-Aba Booster Station Completion 10. Lipede Estate Reticulations 11. Adigbe to Owiwi – 15 kilometers 200mmØ HDPE 12. Owiwi to Aro Lagos Road – 13 kilometers 200mmØHDPE 13. Panseke to Adigbe – 2.5 kilometers – 250mm ØHDPE 14. Pansheke - Onikolobo route – 4 kilometers – 250mmØHDPE 15. Allow for Distribution pipeline within Onikolobo – 8 kilometers – 150mmØ UPVC 16. Allow for Distribution Pipeline within Adigbe – 5 kilometers – 150mmØ 17. Overhead Tank Onikolobo complete with pipeworks – 500m³ 18. Overhead Tank Navy School complete with pipeworks – 500m³ 19. 2 Nos Surface Tanks at Pansheke – 160m³ 20. Obantoko Rising Mains from Iberekodo to Asero – 400mmØ- 10 kilometers (Ductile Iron Pipe) 21. -
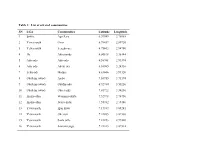
Table 1: List of Selected Communities SN LGA Communities Latitude
Table 1: List of selected communities SN LGA Communities Latitude Longitude 1 Ipokia Ago Sasa 6.59089 2.76065 2 Yewa-south Owo 6.78457 2.89720 3 Yewa-south Ireagbo-are 6.75602 2.94780 4 Ifo Akinsinnde 6.80818 3.16144 5 Ado-odo Ado-odo 6.58768 2.93374 6 Ado-odo Abebi-ota 6.68965 3.24330 7 Ijebu-ode Molipa 6.83606 3.91120 8 Obafemi-owode Ajebo 7.10955 3.71174 9 Obafemi-owode Odofin-odo 6.92744 3.55220 10 Obafemi-owode Oba-seriki 7.01712 3.34230 11 Imeko-afon Wasinmi-okuta 7.52948 2.76750 12 Imeko-afon Iwoye-ketu 7.55782 2.74486 13 Yewa-north Igan ikoto 7.15339 3.04281 14 Yewa-north Oke rori 7.24805 3.02368 15 Yewa-north Saala orile 7.21253 2.97420 16 Yewa-north Araromi joga 7.23323 3.02514 17 Ewekoro Abule Oko 6.86859 3.19430 18 Shagamu Ipoji 6.84440 3.65006 19 Shagamu Odelemo 6.74479 3.66392 20 Ikenne Irolu 6.90834 3.72447 21 Odogbolu Ikosa 6.83873 3.76291 22 Ijebu-east Itele 6.76299 4.06629 23 Ijebu-east Imobi 6.65920 4.17934 24 Ijebu north-east Atan 6.89712 4.00414 25 Abeokuta-south Ibon 7.15864 3.35519 26 Ijebu north Agric 6.93907 3.83253 27 Ijebu north Japara 6.97274 3.99278 28 Remo north Akaka 6.94053 3.71328 29 Odeda Alabata 7.31567 3.53351 30 Odeda Olodo 7.29659 3.60758 31 Abeokuta north Imala odo 7.32122 3.18115 32 Ogun water-side Abigi 6.48618 4.39408 33 Ogun water-side Iwopin 6.51054 4.16990 Table 2: Sex and age distribution of study participants SN LGA Sex (%) Age in years (%) Number Male Female <5yrs 5-15yrs 16-25yrs 26-40yrs 41-70yrs >70yrs Examined 1 Abeokuta north 87 28(32.2) 59(67.8) 7(8.0) 64(7.6) 9(10.3) 3(3.4) 4(4.6) -

The Case of Ifo/Ota Local Government Area of Ogun State, Nigeria
International Journal of Business and Social Science Vol. 5, No. 12; November 2014 Land Market Challenges: The Case of Ifo/Ota Local Government Area of Ogun State, Nigeria S.A. Oloyede, PhD C.A. Ayedun, PhD A.S. Oni, M.Sc. A.O. Oluwatobi, M.Sc. Department of Estate Management, School of Environmental Sciences, College of Science and Technology, Covenant University, Ota, Ogun State, Nigeria Abstract Bearing in mind that land acquisition is very crucial to human development from ages past, the study examined private land acquisition processes and challenges encountered by individuals in Ado-odo/Ota Local government area of Ogun State. Using purposive sampling method, the study gathered relevant data from four different community leaders as well as four heads of family land owners with the aid of questionnaires and employed in- depth interviews to solicit information from eight different youth leaders from the four selected communities. Four each of local artisans (bricklayers, carpenters, plumbers and electricians) available on sites under construction between March and May, 2014 were interviewed on their experiences within the selected neighbourhoods. Data were analyzed using descriptive statistics while percentages and ranking were employed in analysis. Data presentation was basically in tables. The study found that because past governments had failed to take into account the needs and interests of individual, family or community land owners during earlier compulsory land acquisition processes, family land owners are in a hurry to sell off their land even when existing developments are far away. The study recommends that government needs to be proactive in designing new neighbourhood layouts to forestall large informal settlements and, at the same time, implement new methods of financing infrastructure to support urban land development. -

List of Ogirs Tax Stations
LIST OF OGIRS TAX STATIONS S/N TAX OFFICE OFFICE ADDRESS TAX MANAGERS NAMES EMAIL PHONE NOS 1 Oke - Mosan tax Office Internal Revenue Service, Headquaters's Compelx, Oke- osan Abeokuta SEUN OLAJUBE [email protected] 09081705817 2 Akomoje Tax Office Internal Revenue Service,37, Ogunbona Str., Akomoje, Abeokuta LAWAL OLALEKAN [email protected] 09081705796 3 Owode-Egba Tax Office Internal Revenue Service,Obafemi Awolowo Rd., Courtyard building , Owode-Egba ADAMS MUKANDASI AKANBI [email protected] 09081705820 4 Odeda Tax Office Internal Revenue Service, Ibadan Road, Odeda OYEDELE MUYIWA [email protected] 09081705812 5 Adigbe Tax office Internal Revenue Service,Opposite Police Station, Adigbe, Abeokuta ADEESO BOLANLE [email protected] 09081705790 6 Akin-Olugbade Tax Office Internal Revenue Service,Former PHCN Building , Akin Olugbade, Abeokuta BAKARE FATAI [email protected] 09081705795 7 Ota Tax Office Internal Revenue Service,Okesuna B/Stop, Via Fan Mile , Iganmode, Abeokuta JOHN OKHAKUME [email protected] 09081705819 8 Agbara Tax Office Internal Revenue Service,107, Market Rd., Odan, Agbara SABURI OLAJUWON [email protected] 09081705792 9 Ifo Tax Office Internal Revenue Service,Iyana Coker, Ifo ADEGUN MUINAT [email protected] 09081705801 10 Itori Tax Office Internal Revenue Service,Opposite Local Govt., Complex, Itori ADEIGA ADEKUNLE A. [email protected] 09081705811 11 Agbado Tax Office Internal Revenue Service,FRSC Compound, Junction B/Stop, Ijaye Road, Agbado HASSAN KAZEEM [email protected] 09081705791 12 Ijoko Tax Office Internal Revenue Service, Afolabi Olaleye St. Near Salt Company,17, Oke Aro Rd., Agbado KAYODE ODUSANYA [email protected] 09081705804 13 Isheri Tax Office Internal Revenue Service, Gateway City Dev. -
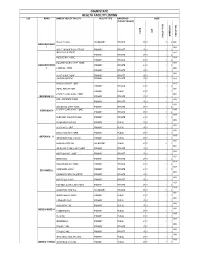
List of Coded Health Facilities in Ogun State.Pdf
OGUN STATE HEALTH FACILITY LISTING LGA WARD NAME OF HEALTH FACILITY FACILITY TYPE OWNERSHIP CODE (PUBLIC/ PRIVATE) LGA STATE OWNERSHIP FACILITY NO FACILITY FACILITY TYPE FACILITY Shallom Hospital SECONDARY PRIVATE 27 01 2 2 0001 IBEREKODO WARD 1 0002 UNCLE JOHN MEDICAL SERVICE PRIMARY PRIVATE 27 01 1 2 INSHA ALAHU EWEJE 0003 PRIMARY PRIVATE 27 01 1 2 MODUPE MAT. HOME 0004 PRIMARY PRIVATE 27 01 1 2 COLUMBIA CLINIC & MAT. HOME 0005 IBEREKODO WARD PRIMARY PRIVATE 27 01 1 2 2 LISABI MAT. HOME 0006 PRIMARY PRIVATE 27 01 1 2 0007 ALASELA MAT. HOME PRIMARY PRIVATE 27 01 1 2 JULIBAM HOSPITAL PRIMARY PRIVATE 27 01 1 2 0008 KING DAVIDS MAT. HOME 0009 PRIMARY PRIVATE 27 01 1 2 ROYAL HEALTH POST 0010 PRIMARY PUBLIC 27 01 1 1 CHARITY CLINIC & MAT. HOME 0011 IBEREKODO 111 PRIMARY PRIVATE 27 01 1 2 ADE – FAITH MAT. HOME 0012 PRIMARY PRIVATE 27 01 1 2 0013 GOD GRACE & MAT. HOME PRIMARY PRIVATE 27 01 1 2 IBEREKODO IV CHARITY CLINIC & MAT. HOME 0014 PRIMARY PRIVATE 27 01 1 2 0015 DURO MED. DIAGNOSTE LAB PRIMARY PRIVATE 27 01 1 2 0016 ELEGA HEALTH CLINIC PRIMARY PUBLIC 27 01 1 1 0017 ALAFIA MAT. HOME PRIMARY PUBLIC 27 01 1 1 0018 OMOLAYAJO MAT. HOME PRIMARY PUBLIC 27 01 1 1 IBEREKODO V 0019 IBEREKODO HEALTH CLINIC PRIMARY PUBLIC 27 01 1 1 0020 GENERAL HOSPITAL SECONDARY PUBLIC 27 01 2 1 0021 IKE-OLUWA CLINIC & MAT. HOME PRIMARY PRIVATE 27 01 1 2 0022 MOPELOLA MAT. HOME PRIMARY PRIVATE 27 01 1 2 0023 BISTED MED PRIMARY PRIVATE 27 01 1 2 0024 TOLUWALASE MAT HOME PRIMARY PRIVATE 27 01 1 2 0025 LADE MAMO.