Xin Et Al., Afr J Tradit Complement Altern Med. (2015) 12(6):39-70
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

-

AGS Seed List No 69 2020
Seed list No 69 2020-21 Garden Collected Seed 1001 Abelia floribunda 1057 Agrostemma githago 1002 Abies koreana 1058 Albuca canadensis (L. -

Fall 2013 NARGS
Rock Garden uar terly � Fall 2013 NARGS to ADVERtISE IN thE QuARtERly CoNtACt [email protected] Let me know what yo think A recent issue of a chapter newsletter had an item entitled “News from NARGS”. There were comments on various issues related to the new NARGS website, not all complimentary, and then it turned to the Quarterly online and raised some points about which I would be very pleased to have your views. “The good news is that all the Quarterlies are online and can easily be dowloaded. The older issues are easy to read except for some rather pale type but this may be the result of scanning. There is amazing information in these older issues. The last three years of the Quarterly are also online but you must be a member to read them. These last issues are on Allen Press’s BrightCopy and I find them harder to read than a pdf file. Also the last issue of the Quarterly has 60 extra pages only available online. Personally I find this objectionable as I prefer all my content in a printed bulletin.” This raises two points: Readability of BrightCopy issues versus PDF issues Do you find the BrightCopy issues as good as the PDF issues? Inclusion of extra material in online editions only. Do you object to having extra material in the online edition which can not be included in the printed edition? Please take a moment to email me with your views Malcolm McGregor <[email protected]> CONTRIBUTORS All illustrations are by the authors of articles unless otherwise stated. -

Karyological Studies on Iris Japonica Thunb. and Its Allies1) 2) by K
180 Cytologia 10 Karyological Studies on Iris japonica Thunb. and Its Allies1) 2) By K. Yasui Tokyo Imperial University (With 8 text figures) Receic ed MTV 28, 1939 Introduction There are several karyological investigations on Iris japonica. KAZAO (1928) considered the sporophyte of this species as an auto triploid having 54 chromosomes. But SIMONET (1932, 1934) re ported I. japonica as a diploid plant with 34 chromosomes in its root-tip cells, and considered I. japonica var aphrodite as a hyper triploid and interpreted the chromosome constitution as 2n=51+3, the latter 3 chromosomes being considered as derived from 3 of 51 chromosomes in I. japonica by fragmentation which occurred att their median constrictions. The karyological investigation in I. japonica and the other 2 allied species, which were kindly put at my deposal by Dr. S. IMA MURA of Kyoto Imp. University, gave me some results different from those of the previous authors as is described below. Material and Method In the fixing of the root-tip cells both NAVASHIN's and LEWITSKY's solutions were used. When fixed in the latter, the chromosomes were found longer and slender than when fixed in the former solution, but there was no essential difference in the distribu tion of chromosomes in the equatorial plate. NEWTON'S gentian violet staining was generally adopted. Materials for the study of meiosis were fixed mostly with NAVASHIN'S fluid and stained with HEIDENHAIN'S iron-alum haematoxylin. Acetocarmine smears were also tried in the study of the pollen mother cells. Drawings were made with a camera lucida. -

Sephadex® LH-20, Isolation, and Purification of Flavonoids from Plant
molecules Review Sephadex® LH-20, Isolation, and Purification of Flavonoids from Plant Species: A Comprehensive Review Javad Mottaghipisheh 1,* and Marcello Iriti 2,* 1 Department of Pharmacognosy, Faculty of Pharmacy, University of Szeged, Eötvös u. 6, 6720 Szeged, Hungary 2 Department of Agricultural and Environmental Sciences, Milan State University, via G. Celoria 2, 20133 Milan, Italy * Correspondence: [email protected] (J.M.); [email protected] (M.I.); Tel.: +36-60702756066 (J.M.); +39-0250316766 (M.I.) Academic Editor: Francesco Cacciola Received: 20 August 2020; Accepted: 8 September 2020; Published: 10 September 2020 Abstract: Flavonoids are considered one of the most diverse phenolic compounds possessing several valuable health benefits. The present study aimed at gathering all correlated reports, in which Sephadex® LH-20 (SLH) has been utilized as the final step to isolate or purify of flavonoid derivatives among all plant families. Overall, 189 flavonoids have been documented, while the majority were identified from the Asteraceae, Moraceae, and Poaceae families. Application of SLH has led to isolate 79 flavonols, 63 flavones, and 18 flavanones. Homoisoflavanoids, and proanthocyanidins have only been isolated from the Asparagaceae and Lauraceae families, respectively, while the Asteraceae was the richest in flavones possessing 22 derivatives. Six flavones, four flavonols, three homoisoflavonoids, one flavanone, a flavanol, and an isoflavanol have been isolated as the new secondary metabolites. This technique has been able to isolate quercetin from 19 plant species, along with its 31 derivatives. Pure methanol and in combination with water, chloroform, and dichloromethane have generally been used as eluents. This comprehensive review provides significant information regarding to remarkably use of SLH in isolation and purification of flavonoids from all the plant families; thus, it might be considered an appreciable guideline for further phytochemical investigation of these compounds. -
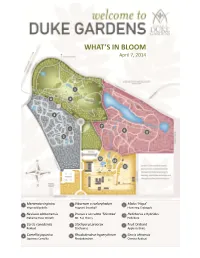
What's in Bloom
WHAT’S IN BLOOM April 7, 2014 5 4 6 2 7 1 9 8 3 12 10 11 1 Mertensia virginica 5 Viburnum x carlcephalum 9 Malus ‘Hopa’ Virginia Bluebells Fragrant Snowball Flowering Crabapple 2 Neviusia alabamensis 6 Prunus x serrulata ‘Shirotae’ 10 Helleborus x hybridus Alabama Snow Wreath Mt. Fuji Cherry Hellebore 3 Cercis canadensis 7 Stachyurus praecox 11 Fruit Orchard Redbud Stachyurus Apple cultivars 4 Camellia japonica 8 Rhododendron hyperythrum 12 Cercis chinensis Japanese Camellia Rhododendron Chinese Redbud WHAT’S IN BLOOM April 7, 2014 BLOMQUIST GARDEN OF NATIVE PLANTS Amelanchier arborea Common Serviceberry Sanguinaria canadensis Bloodroot Cornus florida Flowering Dogwood Stylophorum diphyllum Celandine Poppy Thalictrum thalictroides Rue Anemone Fothergilla major Fothergilla Trillium decipiens Chattahoochee River Trillium Hepatica nobilis Hepatica Trillium grandiflorum White Trillium Hexastylis virginica Wild Ginger Hexastylis minor Wild Ginger Trillium pusillum Dwarf Wakerobin Illicium floridanum Florida Anise Tree Trillium stamineum Blue Ridge Wakerobin Malus coronaria Sweet Crabapple Uvularia sessilifolia Sessileleaf Bellwort Mertensia virginica Virginia Bluebells Pachysandra procumbens Allegheny spurge Prunus americana American Plum DORIS DUKE CENTER GARDENS Camellia japonica Japanese Camellia Pulmonaria ‘Diana Clare’ Lungwort Cercis canadensis Redbud Prunus persica Flowering Peach Puschkinia scilloides Striped Squill Cercis chinensis Redbud Sanguinaria canadensis Bloodroot Clematis armandii Evergreen Clematis Spiraea prunifolia Bridalwreath -

These De Doctorat De L'universite Paris-Saclay
NNT : 2016SACLS250 THESE DE DOCTORAT DE L’UNIVERSITE PARIS-SACLAY, préparée à l’Université Paris-Sud ÉCOLE DOCTORALE N° 567 Sciences du Végétal : du Gène à l’Ecosystème Spécialité de doctorat (Biologie) Par Mlle Nour Abdel Samad Titre de la thèse (CARACTERISATION GENETIQUE DU GENRE IRIS EVOLUANT DANS LA MEDITERRANEE ORIENTALE) Thèse présentée et soutenue à « Beyrouth », le « 21/09/2016 » : Composition du Jury : M., Tohmé, Georges CNRS (Liban) Président Mme, Garnatje, Teresa Institut Botànic de Barcelona (Espagne) Rapporteur M., Bacchetta, Gianluigi Università degli Studi di Cagliari (Italie) Rapporteur Mme, Nadot, Sophie Université Paris-Sud (France) Examinateur Mlle, El Chamy, Laure Université Saint-Joseph (Liban) Examinateur Mme, Siljak-Yakovlev, Sonja Université Paris-Sud (France) Directeur de thèse Mme, Bou Dagher-Kharrat, Magda Université Saint-Joseph (Liban) Co-directeur de thèse UNIVERSITE SAINT-JOSEPH FACULTE DES SCIENCES THESE DE DOCTORAT DISCIPLINE : Sciences de la vie SPÉCIALITÉ : Biologie de la conservation Sujet de la thèse : Caractérisation génétique du genre Iris évoluant dans la Méditerranée Orientale. Présentée par : Nour ABDEL SAMAD Pour obtenir le grade de DOCTEUR ÈS SCIENCES Soutenue le 21/09/2016 Devant le jury composé de : Dr. Georges TOHME Président Dr. Teresa GARNATJE Rapporteur Dr. Gianluigi BACCHETTA Rapporteur Dr. Sophie NADOT Examinateur Dr. Laure EL CHAMY Examinateur Dr. Sonja SILJAK-YAKOVLEV Directeur de thèse Dr. Magda BOU DAGHER KHARRAT Directeur de thèse Titre : Caractérisation Génétique du Genre Iris évoluant dans la Méditerranée Orientale. Mots clés : Iris, Oncocyclus, région Est-Méditerranéenne, relations phylogénétiques, status taxonomique. Résumé : Le genre Iris appartient à la famille des L’approche scientifique est basée sur de nombreux Iridacées, il comprend plus de 280 espèces distribuées outils moléculaires et génétiques tels que : l’analyse de à travers l’hémisphère Nord. -

Isolation, Identification and Characterization of Allelochemicals/Natural Products
Isolation, Identification and Characterization of Allelochemicals/Natural Products Isolation, Identification and Characterization of Allelochemicals/Natural Products Editors DIEGO A. SAMPIETRO Instituto de Estudios Vegetales “Dr. A. R. Sampietro” Universidad Nacional de Tucumán, Tucumán Argentina CESAR A. N. CATALAN Instituto de Química Orgánica Universidad Nacional de Tucumán, Tucumán Argentina MARTA A. VATTUONE Instituto de Estudios Vegetales “Dr. A. R. Sampietro” Universidad Nacional de Tucumán, Tucumán Argentina Series Editor S. S. NARWAL Haryana Agricultural University Hisar, India Science Publishers Enfield (NH) Jersey Plymouth Science Publishers www.scipub.net 234 May Street Post Office Box 699 Enfield, New Hampshire 03748 United States of America General enquiries : [email protected] Editorial enquiries : [email protected] Sales enquiries : [email protected] Published by Science Publishers, Enfield, NH, USA An imprint of Edenbridge Ltd., British Channel Islands Printed in India © 2009 reserved ISBN: 978-1-57808-577-4 Library of Congress Cataloging-in-Publication Data Isolation, identification and characterization of allelo- chemicals/natural products/editors, Diego A. Sampietro, Cesar A. N. Catalan, Marta A. Vattuone. p. cm. Includes bibliographical references and index. ISBN 978-1-57808-577-4 (hardcover) 1. Allelochemicals. 2. Natural products. I. Sampietro, Diego A. II. Catalan, Cesar A. N. III. Vattuone, Marta A. QK898.A43I86 2009 571.9’2--dc22 2008048397 All rights reserved. No part of this publication may be reproduced, stored in a retrieval system, or transmitted in any form or by any means, electronic, mechanical, photocopying or otherwise, without the prior permission of the publisher, in writing. The exception to this is when a reasonable part of the text is quoted for purpose of book review, abstracting etc. -

Phytochemicals
Phytochemicals HO O OH CH OC(CH3)3 3 CH3 CH3 H H O NH O CH3 O O O O OH O CH3 CH3 OH CH3 N N O O O N N CH3 OH HO OH HO Alkaloids Steroids Terpenoids Phenylpropanoids Polyphenols Others Phytochemicals Phytochemical is a general term for natural botanical chemicals Asiatic Acid [A2475] is a pentacyclic triterpene extracted from found in, for example, fruits and vegetables. Phytochemicals are Centella asiatica which is a tropical medicinal plant. Asiatic Acid not necessary for human metabolism, in contrast to proteins, possess wide pharmacological activities. sugars and other essential nutrients, but it is believed that CH3 phytochemicals affect human health. Phytochemicals are CH3 components of herbs and crude drugs used since antiquity by humans, and significant research into phytochemicals continues today. H C CH H C OH HO 3 3 O Atropine [A0754], a tropane alkaloid, was first extracted from H CH3 the root of belladonna (Atropa belladonna) in 1830s. Atropine is a HO competitive antagonist of muscarine-like actions of acetylcholine CH3 H and is therefore classified as an antimuscarinic agent. OH [A2475] O NCH3 O C CHCH2OH Curcumin [C0434] [C2302], a dietary constituent of turmeric, has chemopreventive and chemotherapeutic potentials against various types of cancers. OO CH3O OCH3 [A0754] HO OH Galantamine Hydrobromide [G0293] is a tertiary alkaloid [C0434] [C2302] found in the bulbs of Galanthus woronowi. Galantamine has shown potential for the treatment of Alzheimer's disease. TCI provides many phytochemicals such as alkaloids, steroids, terpenoids, phenylpropanoids, polyphenols and etc. OH References O . HBr Phytochemistry of Medicinal Plants, ed. -

IN SILICO ANALYSIS of FUNCTIONAL Snps of ALOX12 GENE and IDENTIFICATION of PHARMACOLOGICALLY SIGNIFICANT FLAVONOIDS AS
Tulasidharan Suja Saranya et al. Int. Res. J. Pharm. 2014, 5 (6) INTERNATIONAL RESEARCH JOURNAL OF PHARMACY www.irjponline.com ISSN 2230 – 8407 Research Article IN SILICO ANALYSIS OF FUNCTIONAL SNPs OF ALOX12 GENE AND IDENTIFICATION OF PHARMACOLOGICALLY SIGNIFICANT FLAVONOIDS AS LIPOXYGENASE INHIBITORS Tulasidharan Suja Saranya, K.S. Silvipriya, Manakadan Asha Asokan* Department of Pharmaceutical Chemistry, Amrita School of Pharmacy, Amrita Viswa Vidyapeetham University, AIMS Health Sciences Campus, Kochi, Kerala, India *Corresponding Author Email: [email protected] Article Received on: 20/04/14 Revised on: 08/05/14 Approved for publication: 22/06/14 DOI: 10.7897/2230-8407.0506103 ABSTRACT Cancer is a disease affecting any part of the body and in comparison with normal cells there is an elevated level of lipoxygenase enzyme in different cancer cells. Thus generation of lipoxygenase enzyme inhibitors have suggested being valuable. Individual variation was identified by the functional effects of Single Nucleotide Polymorphisms (SNPs). 696 SNPs were identified from the ALOX12 gene, out of which 73 were in the coding non-synonymous region, from which 8 were found to be damaging. In silico analysis was performed to determine naturally occurring flavonoids such as isoflavones having the basic 3- phenylchromen-4-one skeleton for the pharmacological activity, like Genistein, Diadzein, Irilone, Orobol and Pseudobaptigenin. O-methylated isoflavones such as Biochanin, Calycosin, Formononetin, Glycitein, Irigenin, 5-O-Methylgenistein, Pratensein, Prunetin, ψ-Tectorigenin, Retusin and Tectorigenine were also used for the study. Other natural products like Aesculetin, a coumarin derivative; flavones such as ajoene and baicalein were also used for the comparative study of these natural compounds along with acteoside and nordihydroguaiaretic acid (antioxidants) and active inhibitors like Diethylcarbamazine, Zileuton and Azelastine as standard for the computational analysis. -

2016 February Newsletter
PRESCOTT AREA IRIS SOCIETY Calling Card - photo by Carolyn Alexander VOLUME 13 ISSUE 2 FEBRUARY 2016 Presidents Message listJanice of AIS with Display her Gardens. PAIS can take pride in this distinctionname sake, since Janice we will have in Prescott, three of the Greetings to All, onlyChesnik AIS recognized public display gardens in the Happy Winter!! It has been an Southwest. This distinction is due to the dedication of interesting winter with beautiful white the PAIS membership in making each of our projects snow covered mountains, rain, cold and programs a success. From our public gardens to weather and then sunshine with nice our work at the cemetery to our adult and youth warm days. El Nino has been good for education programs the American Iris Society looks at the Prescott area and the state as a PAIS as an example and innovator of what an AIS whole. With the Region 15 Fall affiliate can do to promote iris horticulture across the conference now behind us and a new year of exciting speakers and events Saturday, February 20, 1:30 pm coming up, we look for continued Our first meeting for 2016 features, Janice Chesnik, participation from the PAIS members to and her “Iris War Stories”. We also hope to have our make this another successful year. See our article on page 3 updating our Club Handbook ready for distribution. outreach projects for this year's plans. “My love for irises began when as a child, If you have information or would like to do an article for the newsletter all on Memorial Day (Decoration Day in those older members are welcome to contribute. -

Iris Sibirica and Others Iris Albicans Known As Cemetery
Iris Sibirica and others Iris Albicans Known as Cemetery Iris as is planted on Muslim cemeteries. Two different species use this name; the commoner is just a white form of Iris germanica, widespread in the Mediterranean. This is widely available in the horticultural trade under the name of albicans, but it is not true to name. True Iris albicans which we are offering here occurs only in Arabia and Yemen. It is some 60cm tall, with greyish leaves and one to three, strongly and sweetly scented, 9cm flowers. The petals are pure, bone- white. The bracts are pale green. (The commoner interloper is found across the Mediterranean basin and is not entitled to the name, which continues in use however. The wrongly named albicans, has brown, papery bracts, and off-white flowers). Our stock was first found near Sana’a, Yemen and is thriving here, outside, in a sunny, raised bed. Iris Sibirica and others Iris chrysographes Black Form Clumps of narrow, iris-like foliage. Tall sprays of darkest violet to almost black velvety flowers, Jun-Sept. Ht 40cm. Moist, well drained soil. Part shade. Deepest Purple which is virtually indistinguishable from black. Moist soil. Ht. 50cm Iris chrysographes Dykes (William Rickatson Dykes, 1911, China); Section Limniris, Series Sibericae; 14-18" (35-45 cm), B7D; Flowers dark reddish violet with gold streaks in the signal area giving it its name (golden writing); Collected by E. H. Wilson in 1908, in China; The Gardeners' Chronicle 49: 362. 1911. The Curtis's Botanical Magazine. tab. 8433 in 1912, gives the following information along with the color illustration.