A Randomized Controlled Trial of Ganaxolone in Posttraumatic Stress Disorder
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Exploring Matrix Effects on Binding Properties and Characterization of Cotinine Molecularly Imprinted Polymer on Paper-Based Scaffold
Article Exploring Matrix Effects on Binding Properties and Characterization of Cotinine Molecularly Imprinted Polymer on Paper-Based Scaffold Nutcha Larpant 1, Yaneenart Suwanwong 2, Somchai Boonpangrak 3 and Wanida Laiwattanapaisal 4,5,* 1 Graduate Program in Clinical Biochemistry and Molecular Medicine, Faculty of Allied Health Sciences, Chulalongkorn University, Bangkok 10330, Thailand; [email protected] 2 Department of Clinical Microscopy, Faculty of Allied Health Sciences, Chulalongkorn University, Bangkok 10330, Thailand; [email protected] 3 Center for Research and Innovation, Faculty of Medical Technology, Mahidol University, Nakhon Pathom 73170, Thailand; [email protected] 4 Department of Clinical Chemistry, Faculty of Allied Health Sciences, Chulalongkorn University, Bangkok 10330, Thailand 5 Electrochemistry and Optical Spectroscopy Center of Excellence, Chulalongkorn University, Bangkok 10330, Thailand * Correspondence: [email protected] Received: 22 January 2019; Accepted: 20 March 2019; Published: 26 March 2019 Abstract: Commercially available sorbent materials for solid-phase extraction are widely used in analytical laboratories. However, non-selective binding is a major obstacle for sample analysis. To overcome this problem, molecularly imprinted polymers (MIPs) were used as selective adsorbent materials prior to determining target analysts. In this study, the use of non-covalent molecularly imprinted polymers (MIPs) for cotinine adsorption on a paper-based scaffold was studied. Fiberglass paper was used as a paper scaffold for cotinine-selective MIP adsorption with the use of 0.5% agarose gel. The effects of salt, pH, sample matrix, and solvent on the cotinine adsorption and extraction process were investigated. Under optimal conditions, the adsorption isotherm of synthesized MIPs increased to 125.41 µg/g, whereas the maximum adsorption isotherm of non-imprinted polymers (NIPs) was stable at 42.86 µg/g. -

NINDS Custom Collection II
ACACETIN ACEBUTOLOL HYDROCHLORIDE ACECLIDINE HYDROCHLORIDE ACEMETACIN ACETAMINOPHEN ACETAMINOSALOL ACETANILIDE ACETARSOL ACETAZOLAMIDE ACETOHYDROXAMIC ACID ACETRIAZOIC ACID ACETYL TYROSINE ETHYL ESTER ACETYLCARNITINE ACETYLCHOLINE ACETYLCYSTEINE ACETYLGLUCOSAMINE ACETYLGLUTAMIC ACID ACETYL-L-LEUCINE ACETYLPHENYLALANINE ACETYLSEROTONIN ACETYLTRYPTOPHAN ACEXAMIC ACID ACIVICIN ACLACINOMYCIN A1 ACONITINE ACRIFLAVINIUM HYDROCHLORIDE ACRISORCIN ACTINONIN ACYCLOVIR ADENOSINE PHOSPHATE ADENOSINE ADRENALINE BITARTRATE AESCULIN AJMALINE AKLAVINE HYDROCHLORIDE ALANYL-dl-LEUCINE ALANYL-dl-PHENYLALANINE ALAPROCLATE ALBENDAZOLE ALBUTEROL ALEXIDINE HYDROCHLORIDE ALLANTOIN ALLOPURINOL ALMOTRIPTAN ALOIN ALPRENOLOL ALTRETAMINE ALVERINE CITRATE AMANTADINE HYDROCHLORIDE AMBROXOL HYDROCHLORIDE AMCINONIDE AMIKACIN SULFATE AMILORIDE HYDROCHLORIDE 3-AMINOBENZAMIDE gamma-AMINOBUTYRIC ACID AMINOCAPROIC ACID N- (2-AMINOETHYL)-4-CHLOROBENZAMIDE (RO-16-6491) AMINOGLUTETHIMIDE AMINOHIPPURIC ACID AMINOHYDROXYBUTYRIC ACID AMINOLEVULINIC ACID HYDROCHLORIDE AMINOPHENAZONE 3-AMINOPROPANESULPHONIC ACID AMINOPYRIDINE 9-AMINO-1,2,3,4-TETRAHYDROACRIDINE HYDROCHLORIDE AMINOTHIAZOLE AMIODARONE HYDROCHLORIDE AMIPRILOSE AMITRIPTYLINE HYDROCHLORIDE AMLODIPINE BESYLATE AMODIAQUINE DIHYDROCHLORIDE AMOXEPINE AMOXICILLIN AMPICILLIN SODIUM AMPROLIUM AMRINONE AMYGDALIN ANABASAMINE HYDROCHLORIDE ANABASINE HYDROCHLORIDE ANCITABINE HYDROCHLORIDE ANDROSTERONE SODIUM SULFATE ANIRACETAM ANISINDIONE ANISODAMINE ANISOMYCIN ANTAZOLINE PHOSPHATE ANTHRALIN ANTIMYCIN A (A1 shown) ANTIPYRINE APHYLLIC -

Mechanisms of Action of Antiepileptic Drugs
Review Mechanisms of action of antiepileptic drugs Epilepsy affects up to 1% of the general population and causes substantial disability. The management of seizures in patients with epilepsy relies heavily on antiepileptic drugs (AEDs). Phenobarbital, phenytoin, carbamazepine and valproic acid have been the primary medications used to treat epilepsy for several decades. Since 1993 several AEDs have been approved by the US FDA for use in epilepsy. The choice of the AED is based primarily on the seizure type, spectrum of clinical activity, side effect profile and patient characteristics such as age, comorbidities and concurrent medical treatments. Those AEDs with broad- spectrum activity are often found to exert an action at more than one molecular target. This article will review the proposed mechanisms of action of marketed AEDs in the US and discuss the future of AEDs in development. 1 KEYWORDS: AEDs anticonvulsant drugs antiepileptic drugs epilepsy Aaron M Cook mechanism of action seizures & Meriem K Bensalem-Owen† The therapeutic armamentarium for the treat- patients with refractory seizures. The aim of this 1UK HealthCare, 800 Rose St. H-109, ment of seizures has broadened significantly article is to discuss the past, present and future of Lexington, KY 40536-0293, USA †Author for correspondence: over the past decade [1]. Many of the newer AED pharmacology and mechanisms of action. College of Medicine, Department of anti epileptic drugs (AEDs) have clinical advan- Neurology, University of Kentucky, 800 Rose Street, Room L-455, tages over older, so-called ‘first-generation’ First-generation AEDs Lexington, KY 40536, USA AEDs in that they are more predictable in their Broadly, the mechanisms of action of AEDs can Tel.: +1 859 323 0229 Fax: +1 859 323 5943 dose–response profile and typically are associ- be categorized by their effects on the neuronal [email protected] ated with less drug–drug interactions. -

Effect of Varenicline Combined with Medical Management on Alcohol Use Disorder with Comorbid Cigarette Smoking
Table of Contents I. Summary of Changes to the Statistical Plan …………………. 2. II. Summary of Changes to the Protocol………………………… 2. III. Original Protocol at Trial Initiation ………………………… 5. 1 Downloaded From: https://jamanetwork.com/ on 09/28/2021 I. Summary of changes to the statistical analysis plan The original statistical analysis plan is described in the original protocol below beginning on page 20. In the original statistical analysis plan the stratification factor sex was omitted unintentionally from the primary model specification for the main outcome (percent heavy drinking days, PHDD) although sex was identified as a potential moderator in an exploratory aim. At the analysis stage, we included both stratification factors (site and sex) and their interactions with treatment in the model for PHDD consistent with prior hypothesis that men and women differ in their smoking and drinking behavior and treatment response, and with good statistical practice. Baseline percent heavy drinking days was specified as a covariate in the original analysis plan but in response to comments by the statistical reviewer was dropped as a covariate and included in the final mixed model analyses reported in the paper. In order to make the baseline and end-point measures comparable, baseline percent heavy drinking days and end-point percent heavy drinking days were both summarized over 8 week periods. This 8-week duration was pre-planned for the end-point measure but modified from the original intention to use 30 days of the baseline period. Unstructured variance-covariance matrix was used for the errors since variances at baseline and end-point were different. -

8Th European Congress on Epileptology, Berlin, Germany, 21 – 25 September 2008
Epilepsia, 50(Suppl. 4): 2–262, 2009 doi: 10.1111/j.1528-1167.2009.02063.x 8th ECE PROCEEDINGS 8th European Congress on Epileptology, Berlin, Germany, 21 – 25 September 2008 Sunday 21 September 2008 KV7 channels (KV7.1-5) are encoded by five genes (KCNQ1-5). They have been identified in the last 10–15 years by discovering the caus- 14:30 – 16:00 ative genes for three autosomal dominant diseases: cardiac arrhythmia Hall 1 (long QT syndrome, KCNQ1), congenital deafness (KCNQ1 and KCNQ4), benign familial neonatal seizures (BFNS, KCNQ2 and VALEANT PHARMACEUTICALS SATELLITE SYM- KCNQ3), and peripheral nerve hyperexcitability (PNH, KCNQ2). The fifth member of this gene family (KCNQ5) is not affected in a disease so POSIUM – NEURON-SPECIFIC M-CURRENT K+ CHAN- far. The phenotypic spectrum associated with KCNQ2 mutations is prob- NELS: A NEW TARGET IN MANAGING EPILEPSY ably broader than initially thought (i.e. not only BFNS), as patients with E. Perucca severe epilepsies and developmental delay, or with Rolando epilepsy University of Pavia, Italy have been described. With regard to the underlying molecular pathophys- iology, it has been shown that mutations in KCNQ2 and KCNQ3 Innovations in protein biology, coupled with genetic manipulations, have decrease the resulting K+ current thereby explaining the occurrence of defined the structure and function of many of the voltage- and ligand- epileptic seizures by membrane depolarization and increased neuronal gated ion channels, channel subunits, and receptors that are the underpin- firing. Very subtle changes restricted to subthreshold voltages are suffi- nings of neuronal hyperexcitability and epilepsy. Of the currently cient to cause BFNS which proves in a human disease model that this is available antiepileptic drugs (AEDs), no two act in the same way, but all the relevant voltage range for these channels to modulate the firing rate. -

OPRM1 Genetic Polymorphisms Are Associated with the Plasma Nicotine Metabolite Cotinine Concentration in Methadone Maintenance Patients: a Cross Sectional Study
Journal of Human Genetics (2013) 58, 84–90 & 2013 The Japan Society of Human Genetics All rights reserved 1434-5161/13 www.nature.com/jhg ORIGINAL ARTICLE OPRM1 genetic polymorphisms are associated with the plasma nicotine metabolite cotinine concentration in methadone maintenance patients: a cross sectional study Yu-Ting Chen1, Hsiao-Hui Tsou2, Hsiang-Wei Kuo1, Chiu-Ping Fang1, Sheng-Chang Wang1, Ing-Kang Ho1,3,4, Yao-Sheng Chang1, Chia-Hui Chen1, Chin-Fu Hsiao2, Hsiao-Yu Wu2, Keh-Ming Lin1, Andrew CH Chen5, Jyy-Jih Tsai-Wu6 and Yu-Li Liu1,7,8 Majority of the heroin-dependent patients smoke cigarettes. Although it has been reported that the OPRM1 genetic polymorphism is associated with the brain mu-opioid receptor binding potential in cigarette smokers, there is no direct evidence showing the impact of plasma cotinine, a nicotine metabolite, on treatment responses to methadone maintenance treatment (MMT). In this study, we aimed to test the hypothesis that the genetic polymorphisms in the OPRM1 are associated with the methadone treatment responses and the severity of cigarette smoking directly measured by the plasma concentration of cotinine in a Taiwanese MMT cohort. Fifteen OPRM1 single-nucleotide polymorphisms (SNPs) were selected and genotyped on DNA samples of 366 MMT patients. Plasma concentrations of cotinine were measured by cotinine enzyme-linked immunosorbent assay. The plasma cotinine concentration had positive correlation with concentrations of methadone (P ¼ 0.042) and its metabolite 2-ethylidene-1,5-dimethyl-3,3-diphenyl-pyrrolidine (P ¼ 0.037). Methadone treatment non-responders, defined by a positive urine morphine test, had a higher plasma concentration of cotinine (P ¼ 0.005), but a lower plasma concentration-to- dose ratio of both R- and S-methadone (P ¼ 0.001 and 0.012, respectively) than the responders. -

Nicotine Metabolism and CYP2D6 Phenotype in Smokers
Vol. 10, 261–263, March 2001 Cancer Epidemiology, Biomarkers & Prevention 261 Short Communication Nicotine Metabolism and CYP2D6 Phenotype in Smokers Neil E. Caporaso,1 Caryn Lerman, Janet Audrain, CYP2D6 (debrisoquine hydroxylase) has been studied as a Neil R. Boyd, David Main, Haleem J. Issaq, putative lung cancer susceptibility factor, and it has been pro- Bill Utermahlan, Roni T. Falk, and Peter Shields posed that this enzyme may contribute to the metabolism of Division of Cancer Epidemiology and Genetics, National Cancer Institute, nicotine, thereby influencing the amount a person smokes and Bethesda, Maryland 20892 [N. E. C., R. T. F.]; Lombardi Cancer Research the delivery of carcinogens. Some in vitro (1) and population Center, Georgetown University, Washington, D.C. 20007 [C. L., J. A., (2) studies have suggested a role for CYP2D6 in nicotine N. R. B., D. M.]; Laboratory of Chemical Synthesis and Analysis, Frederick metabolism, although recent work indicates CYP2A6 is the Cancer Research Center, Frederick, Maryland 21701 [H. J. I., B. U.]; and Laboratory of Human Carcinogenesis, Division of Cancer Etiology, NIH, most important P-450 in nicotine metabolism (3, 4). The National Cancer Institute, Bethesda, Maryland 20892 [P. S.] CYP2D6 MP2 is determined by administering DM (5) and determining the ratio of urinary metabolites. A low ratio of unchanged drug:metabolite identifies extensive metabolizers, Abstract whereas PMs exhibit a high ratio. The hypothesis that a poly- We tested the hypothesis that the polymorphic enzyme morphic gene influences nicotine disposition is important be- CYP2D6 is related to nicotine metabolism in 261 healthy cause addicted smokers engage in smoking behavior in such a subjects enrolling in a smoking cessation clinic. -
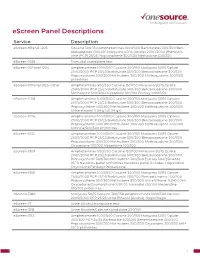
Escreen Panel Descriptions
eScreen Panel Descriptions Service Description eScreen-9Panel-1205 Cocaine 300/150 Amphetamines 1000/500 Barbiturates 300/300 Ben- zodiazepines 300/300 Marijuana 50/15 Opiates 2000/2000 Phencycli- dine (PCP) 25/25 Propoxyphene 300/300 Methadone 300/300 eScreen-1065 Tramadol standalone test eScreen-10Panel-1204 Amphetamines 1000/500 Cocaine 300/150 Marijuana 50/15 Opiate 2000/2000 PCP 25/25 Barbiturate 300/300 Benzodiazepine 300/300 Propoxyphene 300/300 Methadone 300/300 Methaqualone 300/300 pH/nitrites eScreen-10Panel-3125-Other Amphetamines 500/250 Cocaine 150/100 Marijuana 50/15 Opiate 2000/2000 PCP 25/25 Barbiturate 300/300 Benzodiazepine 300/300 Methadone 300/300 Oxycodone 100/100 Ecstasy 1000/250 eScreen-1208 Amphetamines 1000/500 Cocaine 300/150 Marijuana 50/15 Opiates 2000/2000 PCP 25/25 Barbiturate 300/300 Benzodiazepine 300/300 Propoxyphene 300/300 Methadone 300/300 Methaqualone 300/300 Urine ethanol 0.04 g%/0.04 g% eScreen-1224 Amphetamines 1000/500 Cocaine 300/150 Marijuana 50/15 Opiates 2000/2000 PCP 25/25 Barbiturate 300/300 Benzodiazepine 300/300 Propoxyphene 300/300 Methadone 300/300 Methaqualone 300/300 Cotinine 500/500 pH/nitrites eScreen-1232 Amphetamines 1000/500 Cocaine 300/150 Marijuana 50/15 Opiate 2000/2000 PCP 25/25 Barbiturate 300/300 Benzodiazepine 300/300 Propoxyphene 300/300 Methadone 300/300 Methaqualone 300/300 Oxycodone 100/100 Meperidine 100/100 eScreen-1309 Amphetamines 500/250 Cocaine 150/100 Marijuana 50/15 Opiate 2000/2000 PCP 25/25 Barbiturate 300/300 Benzodiazepine 300/300 Propoxyphene 300/300 Methadone -

Ganaxolone (Epilepsy) – Forecast and Market Analysis to 2022
Ganaxolone (Epilepsy) – Forecast and Market Analysis to 2022 Reference Code: GDHC1071DFR Publication Date: February 2013 Executive Summary Epilepsy: Key Metrics in the Epilepsy Markets The below figure illustrates ganaxolone sales for the US 2022 Market Sales and 5EU during the forecast period. US $43.17m Sales for Ganaxolone by Region, 2022 5EU $4.64m Total $47.81m 10% 2012 Total: $47.81m Key Events (2012–2022) Level of Impact Launch of ganaxolone in the US in 2019 ↑↑↑ US Launch of ganaxolone in the 5EU in 2019 ↑↑↑ 5EU Source: GlobalData Sales for Ganaxolone in the Epilepsy Market GlobalData expects Marinus Pharmaceuticals to launch 90% ganaxolone in the US and EU in 2019. We estimate that 2022 sales of ganaxolone will reach $47.81m across Source: GlobalData these markets. Key factors affecting the uptake of ganaxolone will include: What Do the Physicians Think? Novel mechanism of action and good tolerability Overall physicians expressed a need for more AEDs profile and favorable opinions of those in pipeline Efficacy profile is not significantly different from that development. of other marketed anti-epileptic drugs (AEDs) “Among intractable epilepsy patients, any drug that helps Ganaxolone is still in early development; possible treat an additional segment of them will be used, and lack of funding to conduct Phase III trials necessary because we don’t have a basis for using one or another, for commercialization if it’s attractive, it will be used more.” [US] key opinion leader, November 2012 Heavy competition in the market “Brivaracetam is an interesting concept because it’s supposed to be “Super Keppra,” the follow-on from Keppra. -

Chronic Smokeless Tobacco Consumption Contributes to the Development of Renal Diseases in the Human Male Volunteers
Journal of Analytical & Pharmaceutical Research Research Article Open Access Chronic smokeless tobacco consumption contributes to the development of renal diseases in the human male volunteers Abstract Volume 7 Issue 6 - 2018 Smokeless tobacco may able to induce microalbuminuria. The present study S Fareeda Begum,1 G Nagajothi,2 K investigates the relation between nicotine and cotinine with renal function in gutkha Swarnalatha,1 C Suresh Kumar,1 Narendra and khaini users. Methods: The levels of nicotine and cotinine were estimated by HPLC 1 methods and other urine variables were detected by spectrophotometric methods. Maddu 1 Current smokeless tobacco users have shown that significantly elevated levels of Department of Biochemistry, Sri Krishnadevaraya University, India nicotine, cotinine, and epinephrine excretion in the urine than non-tobacco users. 2Department of Corporate Secretaryship, Queen Mary’s Renal function was assessed by glomerular filtration rate (GFR), levels of urea, and College (Autonomous), India creatinine. Among the kidney function measures that we examined, microalbuminuria, decreased glomerular filtration rate, and creatinine clearance were found associated Correspondence: Narendra Maddu, Assistant Professor, with gutkha and khaini users. Significantly decreased proteinuria, urea and increased Department of Biochemistry, Sri Krishnadevaraya University, levels of uric acid and creatinine excretion with the concomitant increase in plasma Ananthapuramu-515003, Andhra Pradesh, India, Tel 91+ total proteins, urea, and decreased uric acid levels were observed in the group I and 9441983797, Email group II users compared to group III users. The products of smokeless tobacco are regarded as good predictors of assessing the free radical levels in the cells. The active Received: June 01, 2018 | Published: November 26, 2018 markers of nitroxidative stress have been elevated progressively with the uptake of nicotine and exposure. -

Molecular Mechanisms of Antiseizure Drug Activity at GABAA Receptors
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Elsevier - Publisher Connector Seizure 22 (2013) 589–600 Contents lists available at SciVerse ScienceDirect Seizure jou rnal homepage: www.elsevier.com/locate/yseiz Review Molecular mechanisms of antiseizure drug activity at GABAA receptors L. John Greenfield Jr.* Dept. of Neurology, University of Arkansas for Medical Sciences, 4301W. Markham St., Slot 500, Little Rock, AR 72205, United States A R T I C L E I N F O A B S T R A C T Article history: The GABAA receptor (GABAAR) is a major target of antiseizure drugs (ASDs). A variety of agents that act at Received 6 February 2013 GABAARs s are used to terminate or prevent seizures. Many act at distinct receptor sites determined by Received in revised form 16 April 2013 the subunit composition of the holoreceptor. For the benzodiazepines, barbiturates, and loreclezole, Accepted 17 April 2013 actions at the GABAAR are the primary or only known mechanism of antiseizure action. For topiramate, felbamate, retigabine, losigamone and stiripentol, GABAAR modulation is one of several possible Keywords: antiseizure mechanisms. Allopregnanolone, a progesterone metabolite that enhances GABAAR function, Inhibition led to the development of ganaxolone. Other agents modulate GABAergic ‘‘tone’’ by regulating the Epilepsy synthesis, transport or breakdown of GABA. GABAAR efficacy is also affected by the transmembrane Antiepileptic drugs chloride gradient, which changes during development and in chronic epilepsy. This may provide an GABA receptor Seizures additional target for ‘‘GABAergic’’ ASDs. GABAAR subunit changes occur both acutely during status Chloride channel epilepticus and in chronic epilepsy, which alter both intrinsic GABAAR function and the response to GABAAR-acting ASDs. -

Ketamine Homogeneous Enzyme Immunoassay (HEIA™)
Ketamine Homogeneous Enzyme Immunoassay (HEIA™) Exclusively from Immunalysis Formula: C13H16CINO Semi-Quantitative or Qualitative Testing Systematic Name: (RS)- 2- (2- chlorophenyl)- 2- (methylamino)cyclohexanone Accurate and reliable Brand Names: Ketanest®, Ketaset®, Ketalar® Ready to use About Ketamine: Ketamine is an anesthetic agent used in the United States since 1972 for veterinary and pediatric medicine. It is also used in the treatment of depression and postoperative pain management. However, in recent years it has gained popularity as a street drug used at clubs and raves due to its hallucinogenic effects. Administration: Oral; intravenous; intramuscular; insufflation Elimination: Ketamine metabolizes by N-demethylation to Norketamine and further dehydrogenates to Dehydronorketamine. After 72 hours of a single dose, 2.3% of Ketamine is unchanged, 1.6% is Norketamine, 16.2% is Dehydronorketamine, and 80% is hydroxylated derivatives of Ketamine.1,2 Abuse Potential: An overdose can cause unconsciousness and dangerously slowed breathing. 1) R. Baselt, Disposition of Toxic Drugs and Chemicals in Man, Fourth Edition, p. 412-414. 2) K. Moore, J.Skerov, B.Levine, and A.Jacobs, Urine Concentrations of Ketamine and Norketamine Following Illegal Consumption, J.Anal, Toxicol. 25: 583-588 (2001). Ketanest® is a registered trademark of Pfizer, Inc., Ketaset® is a trademark of ZOETIS W LLC., Ketalar® is a trademark of PAR STERILE PRODUCTS, LLC. Tel 909.482.0840 | Toll Free 888.664.8378 | Fax 909.482.0850 ISO13485:2003 www.immunalysis.com CERTIFIED