Introduction to the Invertebrate Larval Biology Workshop: a Brief Background
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Buzzle – Zoology Terms – Glossary of Biology Terms and Definitions Http
Buzzle – Zoology Terms – Glossary of Biology Terms and Definitions http://www.buzzle.com/articles/biology-terms-glossary-of-biology-terms-and- definitions.html#ZoologyGlossary Biology is the branch of science concerned with the study of life: structure, growth, functioning and evolution of living things. This discipline of science comprises three sub-disciplines that are botany (study of plants), Zoology (study of animals) and Microbiology (study of microorganisms). This vast subject of science involves the usage of myriads of biology terms, which are essential to be comprehended correctly. People involved in the science field encounter innumerable jargons during their study, research or work. Moreover, since science is a part of everybody's life, it is something that is important to all individuals. A Abdomen: Abdomen in mammals is the portion of the body which is located below the rib cage, and in arthropods below the thorax. It is the cavity that contains stomach, intestines, etc. Abscission: Abscission is a process of shedding or separating part of an organism from the rest of it. Common examples are that of, plant parts like leaves, fruits, flowers and bark being separated from the plant. Accidental: Accidental refers to the occurrences or existence of all those species that would not be found in a particular region under normal circumstances. Acclimation: Acclimation refers to the morphological and/or physiological changes experienced by various organisms to adapt or accustom themselves to a new climate or environment. Active Transport: The movement of cellular substances like ions or molecules by traveling across the membrane, towards a higher level of concentration while consuming energy. -

Invertebrate Biology Wileyonlinelibrary.Com/Journal/Ivb VOLUME 134 | NUMBER 3 | 2015
ivb_134_3_oc_OC.qxd 8/18/2015 10:23 AM Page 1 Invertebrate Invertebrate Biology wileyonlinelibrary.com/journal/ivb VOLUME 134 | NUMBER 3 | 2015 VOLUME 134 NUMBER 3 2015 | | Biology CONTENTS 181–188 Ultrastructure of the rotifer integument: peculiarities of Sinantherina socialis (Monogononta: Invertebrate Biology Gnesiotrocha) Rick Hochberg, Adele Hochberg, and Courtney Chan 189–202 Linking zebra mussel growth and survival with two cellular stress indicators during chronic VOLUME temperature stress Jennifer A. Jost, Emily N. Soltis, Marshall R. Moyer, and Sarah S. Keshwani 134 203–213 Sex‐specific reproductive investment of summer spawners of Illex argentinus in the southwest | NUMBER Atlantic Dongming Lin, Xinjun Chen, Yong Chen, and Zhou Fang 3 – | 214 230 Immunohistochemical investigations of the development of Scoloplos armiger (“intertidalis clade”) 2015 indicate a paedomorphic origin of Proscoloplos cygnochaetus (Annelida, Orbiniidae) Conrad Helm, Anne Krause, and Christoph Bleidorn 231–241 Effects of tidal height and wave exposure on cirrus and penis morphology of the acorn barnacle Tetraclita stalactifera J. Matthew Hoch and Kevin V. Reyes 242–251 The distribution of cave twilight‐zone spiders depends on microclimatic features and trophic supply Raoul Manenti, Enrico Lunghi, and Gentile Francesco Ficetola 252–259 A non‐destructive tissue sampling technique for holothurians to facilitate extraction of DNA for genetic analysis Samantha J. Nowland, Dean R. Jerry, and Paul C. Southgate 260 Erratum COVER ILLUSTRATION Cave habitats are unusual among terrestrial habitats because most of their trophic resources are ultimately derived by transport from surface communities. The amount and distribution of these resources in caves may have strong effects on the biology of cave-dwelling species. -

Glossary - Zoology - Intro
1 Glossary - Zoology - Intro Abdomen: Posterior part of an arthropoda’ body; in vertebrates: abdomen between thorax and pelvic girdle. Acoelous: See coelom. Amixia: A restriction that prevents general intercrossing in a species leading to inbreeding. Anabiosis: Resuscitation after apparent death. Archenteron: See coelom. Aulotomy: Capacity of separating a limb; followed by regeneration; used also for asexual reproduction; see also fissipary (in echinodermata and platyhelminthes). Basal Lamina: Basal plate of developing neural tube; the noncellular, collagenous layer that separates an epithelium from an underlying layer of tissue; also basal membrane. Benthic: Organisms that live on ocean bottoms. Blastocoel: See coelom. Blastula: Stage of embryonic development, at / near the end of cleavage, preceding gastrulation; generally consisting of a hollow ball of cells (coeloblastula); if no blastoceol is present it is termed stereoblastulae (arise from isolecithal and moderately telolecithal ova); in meroblastic cleavage (only the upper part of the zygote is divided), the blastula consists of a disc of cells lying on top of the yolk mass; the blastocoel is reduced to the space separating the cells from the yolk mass. Blastoporus: The opening into the archenteron (the primitive gastric cavity of the gastrula = gastrocoel) developed by the invagination of the blastula = protostoma. Cephalisation: (Gk. kephale, little head) A type of animal body plan or organization in which one end contains a nerve-rich region and functions as a head. Cilium: (pl. cilia, long eyelash) A short, centriole-based, hairlike organelle: Rows of cilia propel certain protista. Cilia also aid the movement of substances across epithelial surfaces of animal cells. Cleavage: The zygote undergoes a series of rapid, synchronous mitotic divisions; results in a ball of many cells. -

Vertebrates and Invertebrates
Vertebrates and invertebrates The animal kingdom is divided into two groups: vertebrates and invertebrates. Vertebrates have been around for millions of years but have evolved and changed over time. The word vertebrate means “having a backbone." Many animals have backbones. You have a backbone. So does a cow, a whale, a fish, a frog, and a bird. Vertebrates are animals that have a backbone. Most animals have a backbone that is made of bones joined together to form a skeleton. Our skeleton gives us our shape and allows us to move. Mammals, fish, birds, reptiles, and amphibians have Vertebrae backbones so they are all vertebrates. Each bone that makes up the backbone Scientists classify vertebrates into five classes: is called a vertebra. These are the • Mammals building blacks that form the backbone, • Fish also known as the spinal cord. The • Birds vertebrae protect and support your • Reptiles spine. Without a backbone, you would not • Amphibians be able to move any part of your body. Animals without a backbone are called invertebrates. Most The human animals are invertebrates. In fact, 95% of all living creatures backbone has 26 on Earth are invertebrates. vertebrae. Arthropods are the largest group of invertebrates. Insects make up a large part of this group. All insects, such as ladybugs, ants, grasshoppers, and bumblebees have three body sections and six legs. Most arthropods live on land, but some of these fascinating creatures live in water. Lobsters, crabs, and shrimp are arthropods that live in the oceans. Many invertebrates have skeletons on the outside of their A frog only has bodies called exoskeletons. -

Fun Facts: Incredible Invertebrates 40 Minutes Age 7-11
Fun Facts: Incredible Invertebrates 40 minutes Age 7-11 1. Invertebrates are animals that do not have a backbone. Some common invertebrates are worms, snails, corals, sponges, insects, jellyfish, and crustaceans (Brsuca and Brusca, 2003). 2. Invertebrates first evolved during the Cambrian Period, over 540 million years ago (Brusca and Brusca, 2003) 3. Over 95% of the 1.4 million known animal species are invertebrates (Smithsonian, 2018) 4. The largest invertebrate is the colossal squid which can weigh over 500kg and reach lengths of up to 14m (Ravaiolo and Youngster, 2012). 5. Corals are sessile invertebrates, meaning they are fixed in one place and unable to relocate. Corals grow at different rates, with some growing up to 10cm in one year and others only growing 0.3cm in one year (Barnes, 1987). 6. Many species of marine worms, such as feather duster and Christmas tree worms, survive by hiding their body in a tube attached to the reef. They feed by extending their gills into the water and catching phytoplankton and other microscopic organisms in the water column (NOAA, 2020). 7. Sea stars and sea urchins are some important species of invertebrates that have specialized organs called tube feet, which allow them to move and feed. The tube feet have two special types of cells. The first type of cell releases a glue-like substance that allows the tube feet to stick and hold onto different structures. The second type of cell releases a substance that breaks down the glue-like substance and allows the tube feet to release from the structure (Flammang et al., 2005). -

Cnidarian Immunity and the Repertoire of Defense Mechanisms in Anthozoans
biology Review Cnidarian Immunity and the Repertoire of Defense Mechanisms in Anthozoans Maria Giovanna Parisi 1,* , Daniela Parrinello 1, Loredana Stabili 2 and Matteo Cammarata 1,* 1 Department of Earth and Marine Sciences, University of Palermo, 90128 Palermo, Italy; [email protected] 2 Department of Biological and Environmental Sciences and Technologies, University of Salento, 73100 Lecce, Italy; [email protected] * Correspondence: [email protected] (M.G.P.); [email protected] (M.C.) Received: 10 August 2020; Accepted: 4 September 2020; Published: 11 September 2020 Abstract: Anthozoa is the most specious class of the phylum Cnidaria that is phylogenetically basal within the Metazoa. It is an interesting group for studying the evolution of mutualisms and immunity, for despite their morphological simplicity, Anthozoans are unexpectedly immunologically complex, with large genomes and gene families similar to those of the Bilateria. Evidence indicates that the Anthozoan innate immune system is not only involved in the disruption of harmful microorganisms, but is also crucial in structuring tissue-associated microbial communities that are essential components of the cnidarian holobiont and useful to the animal’s health for several functions including metabolism, immune defense, development, and behavior. Here, we report on the current state of the art of Anthozoan immunity. Like other invertebrates, Anthozoans possess immune mechanisms based on self/non-self-recognition. Although lacking adaptive immunity, they use a diverse repertoire of immune receptor signaling pathways (PRRs) to recognize a broad array of conserved microorganism-associated molecular patterns (MAMP). The intracellular signaling cascades lead to gene transcription up to endpoints of release of molecules that kill the pathogens, defend the self by maintaining homeostasis, and modulate the wound repair process. -

ZOOL 4420: Invertebrate Zoology California State University Stanislaus Course Syllabus
ZOOL 4420: Invertebrate Zoology California State University Stanislaus Course Syllabus Instructor: Dr. Ritin Bhaduri Office: 263 Naraghi Hall Phone: (209) 667-3485 Email: [email protected] Office Hours: Monday & Friday 9:00 -11:00 AM, or by appointment. Lectures: MWF 8:00 - 8:50 AM in Rm. N210; Lab: W 9:00 – 11:50 AM in Rm N210 Text: Animal Diversity, 6th ed., (2012) C. P. Hickman, L. S. Roberts, et al. McGraw Hill. Announcements: We will use Moodle as our learning management system. Create a Moodle account (code: zool4420-001) and check for lecture slides, videos, etc. on a regular basis. Introduction: Invertebrates comprise at least 95% of all known animals. From their numbers and diversity alone, it is obvious that invertebrates are incredibly important. They are food for humans and other animals, they cause disease, they pollinate most of the plants we need and use, they affect global climate, some are important with respect to medicine, etc. All people, but especially biologists, need to have a good working knowledge of invertebrates Teaching Philosophy: My teaching philosophy is that I want to share as much knowledge and understanding of the subject with students as possible. To see my students excel and become empowered with the newly acquired knowledge is what I feel teaching is all about. My goal for this course is that all participants learn about and come to appreciate all invertebrate groups. In more detail, this involves learning names and classification, and biology of invertebrates. Course Description: Invertebrate Zoology is a senior-level animal diversity course. It is a 4-unit lecture and laboratory course, with 3 lectures and 1 lab period per week. -
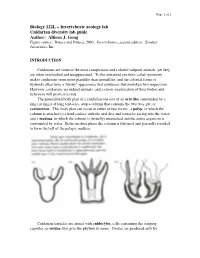
Cnidarians Are Some of the Most Conspicuous and Colorful Tidepool Animals, Yet They Are Often Overlooked and Unappreciated
Page 1 of 1 Biology 122L – Invertebrate zoology lab Cnidarian diversity lab guide Author: Allison J. Gong Figure source: Brusca and Brusca, 2003. Invertebrates, second edition. Sinauer Associates, Inc. INTRODUCTION Cnidarians are some of the most conspicuous and colorful tidepool animals, yet they are often overlooked and unappreciated. To the untrained eye their radial symmetry makes cnidarians seem more plantlike than animallike, and the colonial forms of hydroids often have a "bushy" appearance that reinforces that mistaken first impression. However, cnidarians are indeed animals, and a closer examination of their bodies and behaviors will prove it to you. The generalized body plan of a cnidarian consists of an oral disc surrounded by a ring (or rings) of long tentacles, atop a column that contains the two-way gut, or coelenteron. This body plan can occur in either of two forms: a polyp, in which the column is attached to a hard surface with the oral disc and tentacles facing into the water; and a medusa, in which the column is (usually) unattached and the entire organism is surrounded by water. In the medusa phase the column is flattened and generally rounded to form the bell of the pelagic medusa. Cnidarian tentacles are armed with cnidocytes, cells containing the stinging capsules, or cnidae, that give the phylum its name. Cnidae are produced only by Page 2 of 2 cnidarians, although they can occasionally be found in the cerata of nudibranch molluscs that feed on cnidarians: through a process that is not understood, some nudibranchs are able to ingest cnidae from their prey and sequester them, unfired, in their cerata for defense against their own predators. -

Tropical Marine Invertebrates CAS BI 569 Phylum ANNELIDA by J
Tropical Marine Invertebrates CAS BI 569 Phylum ANNELIDA by J. R. Finnerty Phylum ANNELIDA Porifera Ctenophora Cnidaria Deuterostomia Ecdysozoa Lophotrochozoa Chordata Arthropoda Annelida Hemichordata Onychophora Mollusca Echinodermata Nematoda Platyhelminthes Acoelomorpha Silicispongiae Calcispongia PROTOSTOMIA “BILATERIA” (=TRIPLOBLASTICA) Bilateral symmetry (?) Mesoderm (triploblasty) Phylum ANNELIDA Porifera Ctenophora Cnidaria Deuterostomia Ecdysozoa Lophotrochozoa Chordata Arthropoda Annelida Hemichordata Onychophora Mollusca Echinodermata Nematoda Platyhelminthes Acoelomorpha Silicispongiae Calcispongia PROTOSTOMIA “COELOMATA” True coelom Coelomata gut cavity endoderm mesoderm coelom ectoderm [note: dorso-ventral inversion] Phylum ANNELIDA Porifera Ctenophora Cnidaria Deuterostomia Ecdysozoa Lophotrochozoa Chordata Arthropoda Annelida Hemichordata Onychophora Mollusca Echinodermata Nematoda Platyhelminthes Acoelomorpha Silicispongiae Calcispongia PROTOSTOMIA PROTOSTOMIA “first mouth” blastopore contributes to mouth ventral nerve cord The Blastopore ! Forms during gastrulation ectoderm blastocoel blastocoel endoderm gut blastoderm BLASTULA blastopore The Gut “internal, epithelium-lined cavity for the digestion and absorption of food sponges lack a gut simplest gut = blind sac (Cnidaria) blastopore gives rise to dual- function mouth/anus through-guts evolve later Protostome = blastopore contributes to the mouth Deuterostome = blastopore becomes the anus; mouth is a second opening Protostomy blastopore mouth anus Deuterostomy blastopore -

Invertebrate Paleontology Earth Sciences Division Natural History
Invertebrate Paleontology 19^ Earth Sciences Division Natural History Mu 3311m A NEW SPECIES OF CHIMNEY-BUILDING PENITELLA FROM THE GULF OF ALASKA (BIVALVIA: PHOLADIDAE) * LIBRARY 'Ol ANGBIES GOIJNTV \i: A>., , -APOsrr.'ON GEORGE L. KENNEDY AND JOHN M. ARMENTROUT 1 Made in United States of America Reprinted from THE VELIGER Vol. 32, No. 3, July 1989 © California Malacozoological Society, Inc., 1989 THE VELIGER © CMS, Inc., 1989 The Veliger 32(3):320-325 (July 3, 1989) A New Species of Chimney-Building Penitella from the Gulf of Alaska (Bivalvia: Pholadidae) by GEORGE L. KENNEDY Invertebrate Paleontology Section, Natural History Museum of Los Angeles County, 900 Exposition Boulevard, Los Angeles, California 90007, USA AND JOHN M. ARMENTROUT Mobil Oil Corporation, New Exploration Ventures, P.O. Box 650232, Dallas, Texas 75265, USA Abstract. Penitella hopkinsi sp. nov., from the Gulf of Alaska, differs from other species in the genus by its blunt and thickened posterior margin that inserts against the base of a chimney of agglutinated sediment (unique in the genus), by the shape of the mesoplax, and by details of the umbonal reflection and dorsal extension of the callum. Anatomy is unknown. Late Pleistocene records of the species range from the Seward Peninsula, Alaska, to Point Arena, California. INTRODUCTION acology]); MCZ, Museum of Comparative Zoology, Cam- bridge; NMC, National Museum of Natural Sciences, Recent efforts to identify several enigmatic species of Peni- Ottawa; NSMT, National Science Museum, Tokyo; tella Valenciennes, 1846, from both the northeastern and SBMNH, Santa Barbara Museum of Natural History, northwestern Pacific revealed that a number of nomencla- Santa Barbara; UCLA, University of California, Los An- tural and taxonomic problems in the genus still need to geles (collections at LACM); UCMP, University of Cal- be resolved (KENNEDY, 1985). -

Marine Invertebrate Species Sponges
Marine Invertebrate Species Sponges In Alaskan waters, sponges are primarily subtidal, but some may be found in the intertidal zone. Intertidal sponges usually are inconspicuous, encrusting species growing under ledges, in crevasses, on rocks and boulders. Shades of green, yellow, orange and purple predominate, but other colorations may be found. Sponges have been largely unchanged for 500 million years. Because they taste bad, they have few natural enemies. Some sponges shelter other organisms in their internal cavities. If an intertidal sponge is examined with a magnifying glass, its scattered incurrent and excurrent openings can be seen. Flagellated cells draw sea water into the sponge mass through small incurrent openings. The sea water passes along internal passages and exits the sponge through the larger excurrent openings, which resemble volcanic craters. Inside the sponge mass, the flagellated cells along the passages capture microscopic bits of food from the passing water. Sponges are given shape and texture by fibers and tiny, often elaborate siliceous or calcareous structures called “spicules. Biologists use the size and shape of these spicules to identify sponge species. Some sponges have a distinctive form, which may resemble vases, fingers or balls; but others are amorphous. Sponges produce larvae that drift for a time in the water, then settle to the bottom and stay in the same place to grow and mature. They have no head, eyes, legs or heart. Jellies and Anemones Jellies (often called jellyfish, but they are not fish!) and anemones belong to the same phylum, “Cnidaria," also known as “Coelenterata. In some species, the life cycle of the animal actually includes an alternation of generations: the jellyfishes reproduce sexually, producing larvae that settle to the sea floor and become anemone-like animals. -

IB 103LF: Invertebrate Zoology
IB 103LF: Invertebrate Zoology Course Instructor TBD Graduate Student Instructors TBD COURSE DESCRIPTION AND GOALS Invertebrate zoology includes the biology of all animal organisms that do not have vertebrae, which means more than 95% of all described species of animals. This 5-credit course includes 3h of lecture and 6h of laboratory section per week. Lectures will concentrate on organizing and interpreting information about invertebrates to illustrate (1) evolutionary relationships within and among taxa, (2) morphology, reproduction, development and physiology of major phyla, and (3) adaptations that permit species to inhabit particular environments. Laboratories will be a hands-on opportunity for you to learn about the structure and function of the major invertebrate body plans; and field trips will bring all information together, with living examples. My primary objective in this course is to present the invertebrate diversity that has evolved on Earth (at least of the ones we are aware of); not in depth, but with an overview and selected highlights. By the end of the course you should be able to (1) identify major invertebrate phyla and the morphological characters that define them; (2) apply basic concepts of zoological classification and interpret phylogenetic trees; and (3) discuss current hypotheses for the origin of major invertebrate groups and the relationships among them. Along with introducing you to the diversity and evolution of animal body plans, my goal is also (4) to develop your written and oral communication skills. I also hope you will teach and learn from one another, especially when studying course materials and completing laboratory exercises. ASSIGNMENTS AND GRADING The final grade will be scaled according to course units: 60% for lecture (3 units) and 40% for laboratory (2 units) assignments.