The SEK-1 P38 MAP Kinase Pathway Modulates Gq Signaling in Caenorhabditis Elegans
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Plant Mitogen-Activated Protein Kinase Signaling Cascades Guillaume Tena*, Tsuneaki Asai†, Wan-Ling Chiu‡ and Jen Sheen§
392 Plant mitogen-activated protein kinase signaling cascades Guillaume Tena*, Tsuneaki Asai†, Wan-Ling Chiu‡ and Jen Sheen§ Mitogen-activated protein kinase (MAPK) cascades have components that link sensors/receptors to target genes emerged as a universal signal transduction mechanism that and other cellular responses. connects diverse receptors/sensors to cellular and nuclear responses in eukaryotes. Recent studies in plants indicate that In the past few years, it has become apparent that mitogen- MAPK cascades are vital to fundamental physiological functions activated protein kinase (MAPK) cascades play some of the involved in hormonal responses, cell cycle regulation, abiotic most essential roles in plant signal transduction pathways stress signaling, and defense mechanisms. New findings have from cell division to cell death (Figure 1). MAPK cascades revealed the complexity and redundancy of the signaling are evolutionarily conserved signaling modules with essen- components, the antagonistic nature of distinct pathways, and tial regulatory functions in eukaryotes, including yeasts, the use of both positive and negative regulatory mechanisms. worms, flies, frogs, mammals and plants. The recent enthu- siasm for plant MAPK cascades is backed by numerous Addresses studies showing that plant MAPKs are activated by hor- Department of Molecular Biology, Massachusetts General Hospital, mones, abiotic stresses, pathogens and pathogen-derived Department of Genetics, Harvard Medical School, Wellman 11, elicitors, and are also activated at specific stages during the 50 Blossom Street, Boston, Massachusetts 02114, USA cell cycle [2]. Until recently, studies of MAPK cascades in *e-mail: [email protected] †e-mail: [email protected] plants were focused on cDNA cloning [3,4] and used a ‡e-mail: [email protected] MAPK in-gel assay, MAPK and tyrosine-phosphate anti- §e-mail: [email protected] bodies, and kinase inhibitors to connect signals to MAPKs Current Opinion in Plant Biology 2001, 4:392–400 [2]. -

Autosomal Recessive Ataxias and with the Early-Onset Parkinson’S Disease That We Identified Here As Overlapping with the SFARI Autism Gene
Roadmap to Help Develop Personalized Targeted Treatments for Autism as a Disorder of the Nervous Systems Elizabeth B Torres 1,2,3* 1 Rutgers University Department of Psychology; [email protected] 2 Rutgers University Center for Cognitive Science (RUCCS) 3 Rutgers University Computer Science, Center for Biomedicine Imaging and Modelling (CBIM) * Correspondence: [email protected]; Tel.: (011) +858-445-8909 (E.B.T) 1 | P a g e Supplementary Material Sample Psychological criteria that sidelines sensory motor issues in autism: The ADOS-2 manual [1, 2], under the “Guidelines for Selecting a Module” section states (emphasis added): “Note that the ADOS-2 was developed for and standardized using populations of children and adults without significant sensory and motor impairments. Standardized use of any ADOS-2 module presumes that the individual can walk independently and is free of visual or hearing impairments that could potentially interfere with use of the materials or participation in specific tasks.” Sample Psychiatric criteria from the DSM-5 [3] that does not include sensory-motor issues: A. Persistent deficits in social communication and social interaction across multiple contexts, as manifested by the following, currently or by history (examples are illustrative, not exhaustive, see text): 1. Deficits in social-emotional reciprocity, ranging, for example, from abnormal social approach and failure of normal back-and-forth conversation; to reduced sharing of interests, emotions, or affect; to failure to initiate or respond to social interactions. 2. Deficits in nonverbal communicative behaviors used for social interaction, ranging, for example, from poorly integrated verbal and nonverbal communication; to abnormalities in eye contact and body language or deficits in understanding and use of gestures; to a total lack of facial expressions and nonverbal communication. -

Characterizing Nav1.5 Expression, Organization, and Electrical Behavior in Cardiomyocyte Domains
Graduate School for Cellular and Biomedical Sciences University of Bern Characterizing Nav1.5 expression, organization, and electrical behavior in cardiomyocyte domains PhD Thesis submitted by Sarah Helena Vermij from the Netherlands for the degree of PhD in Biomedical Sciences Supervisor Prof. Dr. Hugues Abriel Institute of Biochemistry and Molecular Medicine Faculty of Medicine of the University of Bern Co-advisor Prof. Dr. Stephan Rohr Department of Physiology Faculty of Medicine of the University of Bern Urheberrechtlicher Hinweis Dieses Dokument steht unter einer Lizenz der Creative Commons Namensnennung-Keine kommerzielle Nutzung-Keine Bearbeitung 2.5 Schweiz. http://creativecommons.org/licenses/by-nc-nd/2.5/ch/ Sie dürfen: dieses Werk vervielfältigen, verbreiten und öffentlich zugänglich machen Zu den folgenden Bedingungen: Namensnennung. Sie müssen den Namen des Autors/Rechteinhabers in der von ihm festgelegten Weise nennen (wodurch aber nicht der Eindruck entstehen darf, Sie oder die Nutzung des Werkes durch Sie würden entlohnt). Keine kommerzielle Nutzung. Dieses Werk darf nicht für kommerzielle Zwecke verwendet werden. Keine Bearbeitung. Dieses Werk darf nicht bearbeitet oder in anderer Weise verändert werden. Im Falle einer Verbreitung müssen Sie anderen die Lizenzbedingungen, unter welche dieses Werk fällt, mitteilen. Jede der vorgenannten Bedingungen kann aufgehoben werden, sofern Sie die Einwilligung des Rechteinhabers dazu erhalten. Diese Lizenz lässt die Urheberpersönlichkeitsrechte nach Schweizer Recht unberührt. Eine ausführliche Fassung des Lizenzvertrags befindet sich unter http://creativecommons.org/licenses/by-nc-nd/2.5/ch/legalcode.de Accepted by the Faculty of Medicine, the Faculty of Science and the Vetsuisse Faculty of the University of Bern at the request of the Graduate School for Cellular and Biomedical Sciences Bern, Dean of the Faculty of Medicine Bern, Dean of the Faculty of Science Bern, Dean of the Vetsuisse Faculty Bern ABSTRACT Proper function of the heart depends on the function of voltage-gated ion channels. -

ERK Cascade Pathway
Biochemistry and Biophysics Reports 7 (2016) 10–19 Contents lists available at ScienceDirect Biochemistry and Biophysics Reports journal homepage: www.elsevier.com/locate/bbrep Glutamine up-regulates MAPK phosphatase-1 induction via activation of Ca2 þ- ERK cascade pathway Otgonzaya Ayush a, Zhe Wu Jin c, Hae-Kyoung Kim b, Yu-Rim Shin d, Suhn-Young Im e, Hern-Ku Lee b,n a Department of Dermatology, Medical University, Mongolian National University of Medical Sciences, Ulaanbaatar, Mongolia b Departments of Immunology and Institute for Medical Science, Chonbuk National University Medical School, Jeonju, Republic of Korea c Department of Anatomy and Histology and Embryology, Yanbian University Medical College, YanJi City, Jilin Province, China d Biofoods Story, Inc, 477 Jeonjucheon-seoro, Wansan-gu, Jeonju, Jeonbuk 560-821, Republic of Korea e Department of Biological Sciences, College of Natural Sciences, Chonnam National University, Gwangju, Republic of Korea article info abstract Article history: The non-essential amino acid L-glutamine (Gln) displays potent anti-inflammatory activity by deacti- Received 3 February 2016 vating p38 mitogen activating protein kinase and cytosolic phospholipase A2 via induction of MAPK Received in revised form phosphatase-1 (MKP-1) in an extracellular signal-regulated kinase (ERK)-dependent way. In this study, 9 May 2016 the mechanism of Gln-mediated ERK-dependency in MKP-1 induction was investigated. Gln increased Accepted 11 May 2016 ERK phosphorylation and activity, and phosphorylations of Ras, c-Raf, and MEK, located in the upstream Available online 12 May 2016 pathway of ERK, in response to lipopolysaccharidein vitro and in vivo. Gln-induced dose-dependent 2 þ Keywords: transient increases in intracellular calcium ([Ca ]i) in MHS macrophage cells. -
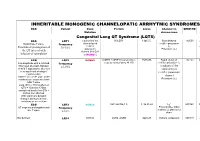
Inheritable Arrhytmia Syndromes
INHERITABLE MONOGENIC CHANNELOPATIC ARRHYTMIC SYNDROMES ECG Variant Gene Protein Locus Channel on OMIM NO IP Mutation chromosome Congenital Long QT Syndrome (LQTS) ECG LQT1 α subunit of the KvLQT1 11p15.5. Slow delayed 192500 AD slow delayed rectifier potassium or Broad base T wave. Frequency rectifier I AR Paradoxical prolongation of 30-35% ks potassium Potassium (IKs) the QT interval with channel (KvLQT1 infusion of epinephrine or KCNQ1) ECG LQT2 KCNH2 (HERG + MiRP1) human ether- 7q35q36 Rapid delayed 152.427 AD a-go-go related gene HERG rectifier potassium I Low-amplitude with a notched, Frequency kr bifurcated alternant, biphasic 25-30% α subunit of the or bifid T appearance due to a rapid delayed very significant slowing of rectifier potassium repolarization. channel KCNH2 on L413P and L559H mutations are associated with Potassium (IKr) bifid T wave Long. QTc > 470ms affected QTc = 450ms to 470ms considered border line QTc < 450ms non affected. With significant dynamic changes during heart rate variations or on exertion + ECG LQT3 SCN5A hH1 and NaV1.5 3, 3p 21-24 INa 600163 AD Prolonged I + influx ST segment prolongation and Frequency Na late T wave. in phase 2, plateau or 5-10% dome Not defined LQT4 KCNJ2 ANK2, ANKB 4q25-27 Sodium, potassium 600919 AR and calcium + Not defined LQT5 KCNE1 minK K Efflux 176261 AD Not defined LQT6 KCNE2 MiRP1 603796 MiRP1 Frequency rare Modest prolongation of QT LQT7 Andersen KCNJ2 Kir2.1 Ik1 Reduction 170390 AD interval, prominent U wave, Great reduction in I Tawil-syndrome k1 frequent PVCs, PVT, result in the bidirectional VT. -

Lipopolysaccharide Macrophage Responses to Tristetraprolin
Dual-Specificity Phosphatase 1 and Tristetraprolin Cooperate To Regulate Macrophage Responses to Lipopolysaccharide This information is current as of August 28, 2019. Tim Smallie, Ewan A. Ross, Alaina J. Ammit, Helen E. Cunliffe, Tina Tang, Dalya R. Rosner, Michael L. Ridley, Downloaded from Christopher D. Buckley, Jeremy Saklatvala, Jonathan L. Dean and Andrew R. Clark J Immunol 2015; 195:277-288; Prepublished online 27 May 2015; http://www.jimmunol.org/ doi: 10.4049/jimmunol.1402830 http://www.jimmunol.org/content/195/1/277 Supplementary http://www.jimmunol.org/content/suppl/2015/05/27/jimmunol.140283 Material 0.DCSupplemental at Aston Univ - SciTech Faculty Team on August 28, 2019 References This article cites 65 articles, 28 of which you can access for free at: http://www.jimmunol.org/content/195/1/277.full#ref-list-1 Why The JI? Submit online. • Rapid Reviews! 30 days* from submission to initial decision • No Triage! Every submission reviewed by practicing scientists • Fast Publication! 4 weeks from acceptance to publication *average Subscription Information about subscribing to The Journal of Immunology is online at: http://jimmunol.org/subscription Permissions Submit copyright permission requests at: http://www.aai.org/About/Publications/JI/copyright.html Email Alerts Receive free email-alerts when new articles cite this article. Sign up at: http://jimmunol.org/alerts The Journal of Immunology is published twice each month by The American Association of Immunologists, Inc., 1451 Rockville Pike, Suite 650, Rockville, MD 20852 Copyright © 2015 The Authors All rights reserved. Print ISSN: 0022-1767 Online ISSN: 1550-6606. The Journal of Immunology Dual-Specificity Phosphatase 1 and Tristetraprolin Cooperate To Regulate Macrophage Responses to Lipopolysaccharide Tim Smallie,*,1 Ewan A. -

Comparative Transcriptome Profiling of the Human and Mouse Dorsal Root Ganglia: an RNA-Seq-Based Resource for Pain and Sensory Neuroscience Research
bioRxiv preprint doi: https://doi.org/10.1101/165431; this version posted October 13, 2017. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. Title: Comparative transcriptome profiling of the human and mouse dorsal root ganglia: An RNA-seq-based resource for pain and sensory neuroscience research Short Title: Human and mouse DRG comparative transcriptomics Pradipta Ray 1, 2 #, Andrew Torck 1 , Lilyana Quigley 1, Andi Wangzhou 1, Matthew Neiman 1, Chandranshu Rao 1, Tiffany Lam 1, Ji-Young Kim 1, Tae Hoon Kim 2, Michael Q. Zhang 2, Gregory Dussor 1 and Theodore J. Price 1, # 1 The University of Texas at Dallas, School of Behavioral and Brain Sciences 2 The University of Texas at Dallas, Department of Biological Sciences # Corresponding authors Theodore J Price Pradipta Ray School of Behavioral and Brain Sciences School of Behavioral and Brain Sciences The University of Texas at Dallas The University of Texas at Dallas BSB 14.102G BSB 10.608 800 W Campbell Rd 800 W Campbell Rd Richardson TX 75080 Richardson TX 75080 972-883-4311 972-883-7262 [email protected] [email protected] Number of pages: 27 Number of figures: 9 Number of tables: 8 Supplementary Figures: 4 Supplementary Files: 6 Word count: Abstract = 219; Introduction = 457; Discussion = 1094 Conflict of interest: The authors declare no conflicts of interest Patient anonymity and informed consent: Informed consent for human tissue sources were obtained by Anabios, Inc. (San Diego, CA). Human studies: This work was approved by The University of Texas at Dallas Institutional Review Board (MR 15-237). -

Dual-Specificity Phosphatases in Immunity and Infection
International Journal of Molecular Sciences Review Dual-Specificity Phosphatases in Immunity and Infection: An Update Roland Lang * and Faizal A.M. Raffi Institute of Clinical Microbiology, Immunology and Hygiene, Universitätsklinikum Erlangen, Friedrich-Alexander-Universität Erlangen-Nürnberg, 91054 Erlangen, Germany * Correspondence: [email protected]; Tel.: +49-9131-85-22979 Received: 15 May 2019; Accepted: 30 May 2019; Published: 2 June 2019 Abstract: Kinase activation and phosphorylation cascades are key to initiate immune cell activation in response to recognition of antigen and sensing of microbial danger. However, for balanced and controlled immune responses, the intensity and duration of phospho-signaling has to be regulated. The dual-specificity phosphatase (DUSP) gene family has many members that are differentially expressed in resting and activated immune cells. Here, we review the progress made in the field of DUSP gene function in regulation of the immune system during the last decade. Studies in knockout mice have confirmed the essential functions of several DUSP-MAPK phosphatases (DUSP-MKP) in controlling inflammatory and anti-microbial immune responses and support the concept that individual DUSP-MKP shape and determine the outcome of innate immune responses due to context-dependent expression and selective inhibition of different mitogen-activated protein kinases (MAPK). In addition to the canonical DUSP-MKP, several small-size atypical DUSP proteins regulate immune cells and are therefore also reviewed here. Unexpected and complex findings in DUSP knockout mice pose new questions regarding cell type-specific and redundant functions. Another emerging question concerns the interaction of DUSP-MKP with non-MAPK binding partners and substrate proteins. -

The ERK MAPK Pathway Modulates Gq-Dependent Locomotion In
bioRxiv preprint doi: https://doi.org/10.1101/231746; this version posted December 10, 2017. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC 4.0 International license. 1 The ERK MAPK pathway modulates Gq-dependent locomotion in 2 Caenorhabditis elegans 3 4 5 Brantley Coleman, Irini Topalidou, and Michael Ailion 6 7 8 9 10 11 12 Department of Biochemistry, University of Washington, Seattle, WA, 98195 13 1 bioRxiv preprint doi: https://doi.org/10.1101/231746; this version posted December 10, 2017. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC 4.0 International license. 14 Running Title: The ERK pathway modulates Gq signaling 15 16 Keywords: Gq signaling, ERK MAPK pathway, Rho small GTPase, C. elegans, G 17 protein 18 19 Corresponding author: 20 Michael Ailion 21 Department of Biochemistry 22 University of Washington 23 Box 357350 24 1705 NE Pacific St 25 Seattle, WA 98195 26 Phone: 206-685-0111 27 email: [email protected] 28 2 bioRxiv preprint doi: https://doi.org/10.1101/231746; this version posted December 10, 2017. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. -

Sodium Channel Mutations in Epilepsy and Other Neurological Disorders
Sodium channel mutations in epilepsy and other neurological disorders Miriam H. Meisler, Jennifer A. Kearney J Clin Invest. 2005;115(8):2010-2017. https://doi.org/10.1172/JCI25466. Review Series Since the first mutations of the neuronal sodium channelS CN1A were identified 5 years ago, more than 150 mutations have been described in patients with epilepsy. Many are sporadic mutations and cause loss of function, which demonstrates haploinsufficiency of SCN1A. Mutations resulting in persistent sodium current are also common. Coding variants of SCN2A, SCN8A, and SCN9A have also been identified in patients with seizures, ataxia, and sensitivity to pain, respectively. The rapid pace of discoveries suggests that sodium channel mutations are significant factors in the etiology of neurological disease and may contribute to psychiatric disorders as well. Find the latest version: https://jci.me/25466/pdf Review series Sodium channel mutations in epilepsy and other neurological disorders Miriam H. Meisler and Jennifer A. Kearney Department of Human Genetics, University of Michigan, Ann Arbor, Michigan, USA. Since the first mutations of the neuronal sodium channel SCN1A were identified 5 years ago, more than 150 muta- tions have been described in patients with epilepsy. Many are sporadic mutations and cause loss of function, which demonstrates haploinsufficiency of SCN1A. Mutations resulting in persistent sodium current are also common. Coding variants of SCN2A, SCN8A, and SCN9A have also been identified in patients with seizures, ataxia, and sen- sitivity to pain, respectively. The rapid pace of discoveries suggests that sodium channel mutations are significant factors in the etiology of neurological disease and may contribute to psychiatric disorders as well. -

Roles of Induced Expression of MAPK Phosphatase-2 in Tumor Development in RET-MEN2A Transgenic Mice
Oncogene (2008) 27, 5684–5695 & 2008 Macmillan Publishers Limited All rights reserved 0950-9232/08 $32.00 www.nature.com/onc ORIGINAL ARTICLE Roles of induced expression of MAPK phosphatase-2 in tumor development in RET-MEN2A transgenic mice T Hasegawa1,2, A Enomoto1,3, T Kato1, K Kawai1, R Miyamoto1, M Jijiwa1, M Ichihara4, M Ishida1, N Asai1, YMurakumo 1, K Ohara1,2, YNiwa 2, H Goto2 and M Takahashi1,5 1Department of Pathology, Nagoya University Graduate School of Medicine, Nagoya, Japan; 2Department of Gastroenterology, Nagoya University Graduate School of Medicine, Nagoya, Japan; 3Institute for Advanced Research, Nagoya University, Nagoya, Japan; 4Department of Biomedical Sciences, College of Life and Health Sciences, Chubu University, Kasugai, Japan and 5Division of Molecular Pathology, Center for Neurological Disease and Cancer, Nagoya University Graduate School of Medicine, Nagoya, Japan Germline mutations in the RET tyrosine kinase gene are line-derived neurotrophic factor (GDNF) family responsible for the development of multiple endocrine of ligands (GFLs). It has a crucial role in transducing neoplasia 2A and 2B (MEN2A and MEN2B). However, growth and differentiation signals in tissues knowledge of the fundamental principles that determine derived from the neural crest and the developing kidney the mutant RET-mediated signaling remains elusive. (Schuchardt et al., 1994; Moore et al., 1996; Pichel Here, we report increased expression of mitogen-activated et al., 1996; Sa´ nchez et al., 1996; Treanor et al., 1996; protein kinase phosphatase-2 (MKP-2) in carcinomas Rosenthal, 1999; Airaksinen and Saarma, 2002). developed in transgenic mice carrying RET with the It has been firmly established that germline mutations MEN2A mutation (RET-MEN2A). -

Dual-Specificity Phosphatases 2
Genes and Immunity (2013) 14, 1–6 & 2013 Macmillan Publishers Limited All rights reserved 1466-4879/13 www.nature.com/gene REVIEW Dual-specificity phosphatases 2: surprising positive effect at the molecular level and a potential biomarker of diseases WWei1,2, Y Jiao2,3, A Postlethwaite4,5, JM Stuart4,5, Y Wang6, D Sun1 and W Gu2 Dual-specificity phosphatases (DUSPs) is an emerging subclass of the protein tyrosine phosphatase gene superfamily, a heterogeneous group of protein phosphatases that can dephosphorylate both phosphotyrosine and phosphoserine/ phosphothreonine residues within the one substrate. Recently, a series of investigations of DUSPs defined their essential roles in cell proliferation, cancer and the immune response. This review will focus on DUSP2, its involvement in different diseases and its potential as a therapeutic target. Genes and Immunity (2013) 14, 1–6; doi:10.1038/gene.2012.54; published online 29 November 2012 Keywords: dual-specificity phosphatases; disease; mitogen-activated protein kinase; immune INTRODUCTION extracellular stimuli. Inducible nucleuses MKPs include DUSP1, Mitogen-activated protein kinase (MAPK) activation cascades DUSP2, DUSP4 and DUSP5, which originated from a common mediate various physiological processes, such as cell proliferation, ancestral gene. They were shown to dephosphorylate Erks, Jnk differentiation, stress responses, inflammation, apoptosis and and p38 MAPKs to the same extent and to be induced by growth immune defense.1–4 Dysregulation of MAPK activation cascades or stress signals. DUSP6, DUSP7 and DUSP9 are cytoplasmic Erk- has been implicated in various diseases and has been the focus of specific MPKs, which can preferentially recognize Erk1 and Erk2 extensive research.5–7 MAPKs are grouped into three major classes in vitro.