Quantum Numbers/Electron Configurations
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Lecture #2: August 25, 2020 Goal Is to Define Electrons in Atoms
Lecture #2: August 25, 2020 Goal is to define electrons in atoms • Bohr Atom and Principal Energy Levels from “orbits”; Balance of electrostatic attraction and centripetal force: classical mechanics • Inability to account for emission lines => particle/wave description of atom and application of wave mechanics • Solutions of Schrodinger’s equation, Hψ = Eψ Required boundaries => quantum numbers (and the Pauli Exclusion Principle) • Electron configurations. C: 1s2 2s2 2p2 or [He]2s2 2p2 Na: 1s2 2s2 2p6 3s1 or [Ne] 3s1 => Na+: [Ne] Cl: 1s2 2s2 2p6 3s23p5 or [Ne]3s23p5 => Cl-: [Ne]3s23p6 or [Ar] What you already know: Quantum Numbers: n, l, ml , ms n is the principal quantum number, indicates the size of the orbital, has all positive integer values of 1 to ∞(infinity) (Bohr’s discrete orbits) l (angular momentum) orbital 0s l is the angular momentum quantum number, 1p represents the shape of the orbital, has integer values of (n – 1) to 0 2d 3f ml is the magnetic quantum number, represents the spatial direction of the orbital, can have integer values of -l to 0 to l Other terms: electron configuration, noble gas configuration, valence shell ms is the spin quantum number, has little physical meaning, can have values of either +1/2 or -1/2 Pauli Exclusion principle: no two electrons can have all four of the same quantum numbers in the same atom (Every electron has a unique set.) Hund’s Rule: when electrons are placed in a set of degenerate orbitals, the ground state has as many electrons as possible in different orbitals, and with parallel spin. -

Principal, Azimuthal and Magnetic Quantum Numbers and the Magnitude of Their Values
268 A Textbook of Physical Chemistry – Volume I Principal, Azimuthal and Magnetic Quantum Numbers and the Magnitude of Their Values The Schrodinger wave equation for hydrogen and hydrogen-like species in the polar coordinates can be written as: 1 휕 휕휓 1 휕 휕휓 1 휕2휓 8휋2휇 푍푒2 (406) [ (푟2 ) + (푆푖푛휃 ) + ] + (퐸 + ) 휓 = 0 푟2 휕푟 휕푟 푆푖푛휃 휕휃 휕휃 푆푖푛2휃 휕휙2 ℎ2 푟 After separating the variables present in the equation given above, the solution of the differential equation was found to be 휓푛,푙,푚(푟, 휃, 휙) = 푅푛,푙. 훩푙,푚. 훷푚 (407) 2푍푟 푘 (408) 3 푙 푘=푛−푙−1 (−1)푘+1[(푛 + 푙)!]2 ( ) 2푍 (푛 − 푙 − 1)! 푍푟 2푍푟 푛푎 √ 0 = ( ) [ 3] . exp (− ) . ( ) . ∑ 푛푎0 2푛{(푛 + 푙)!} 푛푎0 푛푎0 (푛 − 푙 − 1 − 푘)! (2푙 + 1 + 푘)! 푘! 푘=0 (2푙 + 1)(푙 − 푚)! 1 × √ . 푃푚(퐶표푠 휃) × √ 푒푖푚휙 2(푙 + 푚)! 푙 2휋 It is obvious that the solution of equation (406) contains three discrete (n, l, m) and three continuous (r, θ, ϕ) variables. In order to be a well-behaved function, there are some conditions over the values of discrete variables that must be followed i.e. boundary conditions. Therefore, we can conclude that principal (n), azimuthal (l) and magnetic (m) quantum numbers are obtained as a solution of the Schrodinger wave equation for hydrogen atom; and these quantum numbers are used to define various quantum mechanical states. In this section, we will discuss the properties and significance of all these three quantum numbers one by one. Principal Quantum Number The principal quantum number is denoted by the symbol n; and can have value 1, 2, 3, 4, 5…..∞. -

Vibrational Quantum Number
Fundamentals in Biophotonics Quantum nature of atoms, molecules – matter Aleksandra Radenovic [email protected] EPFL – Ecole Polytechnique Federale de Lausanne Bioengineering Institute IBI 26. 03. 2018. Quantum numbers •The four quantum numbers-are discrete sets of integers or half- integers. –n: Principal quantum number-The first describes the electron shell, or energy level, of an atom –ℓ : Orbital angular momentum quantum number-as the angular quantum number or orbital quantum number) describes the subshell, and gives the magnitude of the orbital angular momentum through the relation Ll2 ( 1) –mℓ:Magnetic (azimuthal) quantum number (refers, to the direction of the angular momentum vector. The magnetic quantum number m does not affect the electron's energy, but it does affect the probability cloud)- magnetic quantum number determines the energy shift of an atomic orbital due to an external magnetic field-Zeeman effect -s spin- intrinsic angular momentum Spin "up" and "down" allows two electrons for each set of spatial quantum numbers. The restrictions for the quantum numbers: – n = 1, 2, 3, 4, . – ℓ = 0, 1, 2, 3, . , n − 1 – mℓ = − ℓ, − ℓ + 1, . , 0, 1, . , ℓ − 1, ℓ – –Equivalently: n > 0 The energy levels are: ℓ < n |m | ≤ ℓ ℓ E E 0 n n2 Stern-Gerlach experiment If the particles were classical spinning objects, one would expect the distribution of their spin angular momentum vectors to be random and continuous. Each particle would be deflected by a different amount, producing some density distribution on the detector screen. Instead, the particles passing through the Stern–Gerlach apparatus are deflected either up or down by a specific amount. -

The Quantum Mechanical Model of the Atom
The Quantum Mechanical Model of the Atom Quantum Numbers In order to describe the probable location of electrons, they are assigned four numbers called quantum numbers. The quantum numbers of an electron are kind of like the electron’s “address”. No two electrons can be described by the exact same four quantum numbers. This is called The Pauli Exclusion Principle. • Principle quantum number: The principle quantum number describes which orbit the electron is in and therefore how much energy the electron has. - it is symbolized by the letter n. - positive whole numbers are assigned (not including 0): n=1, n=2, n=3 , etc - the higher the number, the further the orbit from the nucleus - the higher the number, the more energy the electron has (this is sort of like Bohr’s energy levels) - the orbits (energy levels) are also called shells • Angular momentum (azimuthal) quantum number: The azimuthal quantum number describes the sublevels (subshells) that occur in each of the levels (shells) described above. - it is symbolized by the letter l - positive whole number values including 0 are assigned: l = 0, l = 1, l = 2, etc. - each number represents the shape of a subshell: l = 0, represents an s subshell l = 1, represents a p subshell l = 2, represents a d subshell l = 3, represents an f subshell - the higher the number, the more complex the shape of the subshell. The picture below shows the shape of the s and p subshells: (notice the electron clouds) • Magnetic quantum number: All of the subshells described above (except s) have more than one orientation. -

4 Nuclear Magnetic Resonance
Chapter 4, page 1 4 Nuclear Magnetic Resonance Pieter Zeeman observed in 1896 the splitting of optical spectral lines in the field of an electromagnet. Since then, the splitting of energy levels proportional to an external magnetic field has been called the "Zeeman effect". The "Zeeman resonance effect" causes magnetic resonances which are classified under radio frequency spectroscopy (rf spectroscopy). In these resonances, the transitions between two branches of a single energy level split in an external magnetic field are measured in the megahertz and gigahertz range. In 1944, Jevgeni Konstantinovitch Savoiski discovered electron paramagnetic resonance. Shortly thereafter in 1945, nuclear magnetic resonance was demonstrated almost simultaneously in Boston by Edward Mills Purcell and in Stanford by Felix Bloch. Nuclear magnetic resonance was sometimes called nuclear induction or paramagnetic nuclear resonance. It is generally abbreviated to NMR. So as not to scare prospective patients in medicine, reference to the "nuclear" character of NMR is dropped and the magnetic resonance based imaging systems (scanner) found in hospitals are simply referred to as "magnetic resonance imaging" (MRI). 4.1 The Nuclear Resonance Effect Many atomic nuclei have spin, characterized by the nuclear spin quantum number I. The absolute value of the spin angular momentum is L =+h II(1). (4.01) The component in the direction of an applied field is Lz = Iz h ≡ m h. (4.02) The external field is usually defined along the z-direction. The magnetic quantum number is symbolized by Iz or m and can have 2I +1 values: Iz ≡ m = −I, −I+1, ..., I−1, I. -

Quantum Spin Systems and Their Local and Long-Time Properties Carolee Wheeler Faculty Advisor: Robert Sims
URA Project Proposal Quantum Spin Systems and Their Local and Long-Time Properties Carolee Wheeler Faculty Advisor: Robert Sims In quantum mechanics, spin is an important concept having to do with atomic nuclei, hadrons, and elementary particles. Spin may be thought of as a measure of a particle’s rotation about its axis. However, spin differs from orbital angular momentum in the sense that the particles may carry integer or half-integer quantum numbers, i.e. 0, 1/2, 1, 3/2, 2, etc., whereas orbital angular momentum may only take integer quantum numbers. Furthermore, the spin of a charged elementary particle is related to a magnetic dipole moment. All quantum mechanic particles have an inherent spin. This is due to the fact that elementary particles (such as photons, electrons, or quarks) cannot be divided into smaller entities. In other words, they cannot be viewed as particles that are made up of individual, smaller particles that rotate around a common center. Thus, the spin that elementary particles carry is an intrinsic property [1]. An important characteristic of spin in quantum mechanics is that it is quantized. The magnitude of spin takes values S = h s(s + )1 , with h being the reduced Planck’s constant and s being the spin quantum number (a non- negative integer or half-integer). Spin may also be viewed in composite particles, and is calculated by summing the spins of the constituent particles. In the case of atoms and molecules, spin is the sum of the spins of unpaired electrons [1]. Particles with spin can possess a magnetic moment. -

The Principal Quantum Number the Azimuthal Quantum Number The
To completely describe an electron in an atom, four quantum numbers are needed: energy (n), angular momentum (ℓ), magnetic moment (mℓ), and spin (ms). The Principal Quantum Number This quantum number describes the electron shell or energy level of an atom. The value of n ranges from 1 to the shell containing the outermost electron of that atom. For example, in caesium (Cs), the outermost valence electron is in the shell with energy level 6, so an electron incaesium can have an n value from 1 to 6. For particles in a time-independent potential, as per the Schrödinger equation, it also labels the nth eigen value of Hamiltonian (H). This number has a dependence only on the distance between the electron and the nucleus (i.e. the radial coordinate r). The average distance increases with n, thus quantum states with different principal quantum numbers are said to belong to different shells. The Azimuthal Quantum Number The angular or orbital quantum number, describes the sub-shell and gives the magnitude of the orbital angular momentum through the relation. ℓ = 0 is called an s orbital, ℓ = 1 a p orbital, ℓ = 2 a d orbital, and ℓ = 3 an f orbital. The value of ℓ ranges from 0 to n − 1 because the first p orbital (ℓ = 1) appears in the second electron shell (n = 2), the first d orbital (ℓ = 2) appears in the third shell (n = 3), and so on. This quantum number specifies the shape of an atomic orbital and strongly influences chemical bonds and bond angles. -

Chem 1A Chapter 7 Exercises
Chem 1A Chapter 7 Exercises Exercises #1 Characteristics of Waves 1. Consider a wave with a wavelength = 2.5 cm traveling at a speed of 15 m/s. (a) How long does it take for the wave to travel a distance of one wavelength? (b) How many wavelengths (or cycles) are completed in 1 second? (The number of waves or cycles completed per second is referred to as the wave frequency.) (c) If = 3.0 cm but the speed remains the same, how many wavelengths (or cycles) are completed in 1 second? (d) If = 1.5 cm and the speed remains the same, how many wavelengths (or cycles) are completed in 1 second? (e) What can you conclude regarding the relationship between wavelength and number of cycles traveled in one second, which is also known as frequency ()? Answer: (a) 1.7 x 10–3 s; (b) 600/s; (c) 500/s; (d) 1000/s; (e) ?) 2. Consider a red light with = 750 nm and a green light = 550 nm (a) Which light travels a greater distance in 1 second, red light or green light? Explain. (b) Which light (red or green) has the greater frequency (that is, contains more waves completed in 1 second)? Explain. (c) What are the frequencies of the red and green light, respectively? (d) If light energy is defined by the quantum formula, such that Ep = h, which light carries more energy per photon? Explain. 8 -34 (e) Assuming that the speed of light, c = 2.9979 x 10 m/s, Planck’s constant, h = 6.626 x 10 J.s., and 23 Avogadro’s number, NA = 6.022 x 10 /mol, calculate the energy: (i) per photon; (ii) per mole of photon for green and red light, respectively. -
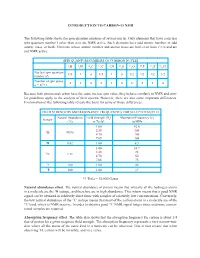
INTRODUCTION to CARBON-13 NMR the Following Table Shows The
INTRODUCTION TO CARBON-13 NMR The following table shows the spin quantum numbers of several nuclei. Only elements that have a nuclear spin quantum number I other than zero are NMR active. Such elements have odd atomic number, or odd atomic mass, or both. Elements whose atomic number and atomic mass are both even have I = 0 and are not NMR active. SPIN QUANTUM NUMBERS OF COMMON NUCLEI 1 2 12 13 14 16 17 19 31 35 1H 1H 6C 6C 7N 8O 8O 9F 15P 17Cl Nuclear spin quantum 1/2 1 0 1/2 1 0 5/2 1/2 1/2 3/2 number (I) Number of spin states 2 3 0 2 3 0 6 2 2 4 n = 2I + 1 Because both proton and carbon have the same nuclear spin value, they behave similarly in NMR and simi- lar guidelines apply to the analysis of their spectra. However, there are also some important differences. Examination of the following table reveals the basis for some of those differences. FIELD STRENGTHS AND RESONANCE FREQUENCIES FOR SELECTED NUCLEI Natural Abundance Field Strength (B ) Absorption Frequency (n) Isotope o (%) in Tesla* in MHz 1.00 42.6 2.35 100 1H 99.98 4.70 200 7.05 300 2H 0.02 1.00 6.5 1.00 10.7 2.35 25 13C 1.11 4.70 50 7.05 75 19F 100 1.00 40 31P 100 1.00 17 *1 Tesla = 10,000 Gauss Natural abundance effect. The natural abundance of proton means that virtually all the hydrogen atoms in a molecule are the 1H isotope, and therefore are in high abundance. -

Free Electron Fermi Gas
Phys463.nb 33 6 Free Electron Fermi Gas 6.1. Electrons in a metal 6.1.1. Electrons in one atom One electron in an atom (a hydrogen-like atom): the nucleon has charge +Z e, where Z is the atomic number, and there is one electron moving around this nucleon Four quantum number: n, l and lz, sz. Energy levels En with n = 1, 2, 3 ... Z2 m e4 1 En = - (6.1) 2 2 2 2 32 p e0 Ñ n where m = me mN me + mN » me where me is the mass of an electron and mN is the mass of the nucleon. 13.6 eV Z2 En = - H L (6.2) n2 H L For each n, the angular momentum quantum number [L2 y = l l + 1 y] can take the values of l = 0, 1, 2, 3, 4 … , n - 1. These states are known as the s, p, d, f , g,… states For each l, the quantum number for Lz can be any integer betweenH -Ll and +l lz = -l, -l + 1,…, l - 1, l . For fixed n, l and lz, the spin quantum number sz can be +1/2 or -1/2 (up or down). H L At each n, there are 2 n2 quantum states. In a real atom In a hydrogen-like atom, for the same n, all the 2 n2 quantum number has the same energy. In a real atom, the energy level splits according to the total angular momentum quantum number l. Typically, the state with lower l has lower energy (not always true). -

Part 1. the Electronic Structure of Atoms Hydrogen-Like Atoms
Part 1. The Electronic Structure of Atoms The wave function of an atom contains all the information about the properties of the atom. The wave functions for the hydrogen atom and other one-electron atoms (such as He+ and Li2+) can be calculated exactly by solving Schrödinger’s equation. Approximate methods must be used to calculate the wave functions for atoms containing two or more electrons. These numerical calculations can be very accurate, but require complicated computer calculations. So we start with (brief review from Chem 331): Hydrogen-Like Atoms What are they? He+ (Z = 2) , Li2+ (Z = 3), Be3+ (Z = 4), etc. Ions with one nucleus and one electron (two bound particles), atomic number Z > 1. Also a few rare, but interesting, “exotics”, such as muonium (more on that in the tutorial!). Why are they important? Schrödinger’s equation can be solved exactly for hydrogen-like atoms, using the reduced mass concept to simplify the two-body problem to an effective one-body problem. This greatly expands the number of chemical systems that can be treated analytically by quantum mechanical principles. (For molecules and multi-electron atoms, cumbersome and less accurate numerical methods must be used to solve Schrödinger’s equation.) For hydrogen-like atoms, the electrostatic potential energy and the reduced mass in Schrödinger’s equation for the hydrogen atom e2 V (r) = − 40r m m = e p me + mp are changed to (notice the factor of Z) Ze 2 V (r) = − 40r m m = e N me + mN me is the electron mass and mN is the nucleus mass. -

Spin Quantum Number Or Rest Angular Momentum
Spin Quantum Number or Rest Angular Momentum H. Razmi (1) and A. MohammadKazemi (2) Department of Physics, The University of Qom, The Old Isfahan Road, Qom, I. R. Iran. (1) [email protected] & [email protected] (2) [email protected] Abstract The aim of this letter (considering the fundamental origins of Klein-Gordon and Dirac equations, Thomas precession, and the photon spin) is to point out that since the origin of spin angular momentum refers to relativity than quantum theory, it is better to call it as the “rest angular momentum”. 1 Spin quantity which was introduced short after formulation of quantum mechanics, in addition to its theoretical significance, has a wide variety of applications in different technological fields (e. g. NMR and new hot subject of research spintronics). Although the mathematical formulation (Lie algebra) of the subject is well-known, the fundamental discussions related to the spin can be hardly found in modern (text)books and papers. In this letter, with an overview of the fundamental origins of Klein-Gordon and Dirac equations, Thomas precession, and the photon spin, it is pointed out that the origin of spin angular momentum refers to relativity than quantum theory. As is clear in the history of the discovery of the electron spin [1], spin degree of freedom cannot be considered as the electron rotation. If the electron is to rotate around itself, its surface velocity should be more than the velocity of light in vacuum?! Although some experts have tried to find a realistic and causal model for spin angular momentum [2], they haven't succeeded to satisfy the physics society yet; the reason is that the so-called classical models are "toy" models than be considered realistic.