Comparative Genomics of the Coconut Crab and Other Decapod Crustaceans
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Development of Species-Specific Edna-Based Test Systems For
REPORT SNO 7544-2020 Development of species-specific eDNA-based test systems for monitoring of non-indigenous Decapoda in Danish marine waters © Henrik Carl, Natural History Museum, Denmark History © Henrik Carl, Natural NIVA Denmark Water Research REPORT Main Office NIVA Region South NIVA Region East NIVA Region West NIVA Denmark Gaustadalléen 21 Jon Lilletuns vei 3 Sandvikaveien 59 Thormøhlensgate 53 D Njalsgade 76, 4th floor NO-0349 Oslo, Norway NO-4879 Grimstad, Norway NO-2312 Ottestad, Norway NO-5006 Bergen Norway DK 2300 Copenhagen S, Denmark Phone (47) 22 18 51 00 Phone (47) 22 18 51 00 Phone (47) 22 18 51 00 Phone (47) 22 18 51 00 Phone (45) 39 17 97 33 Internet: www.niva.no Title Serial number Date Development of species-specific eDNA-based test systems for monitoring 7544-2020 22 October 2020 of non-indigenous Decapoda in Danish marine waters Author(s) Topic group Distribution Steen W. Knudsen and Jesper H. Andersen – NIVA Denmark Environmental monitor- Public Peter Rask Møller – Natural History Museum, University of Copenhagen ing Geographical area Pages Denmark 54 Client(s) Client's reference Danish Environmental Protection Agency (Miljøstyrelsen) UCB and CEKAN Printed NIVA Project number 180280 Summary We report the development of seven eDNA-based species-specific test systems for monitoring of marine Decapoda in Danish marine waters. The seven species are 1) Callinectes sapidus (blå svømmekrabbe), 2) Eriocheir sinensis (kinesisk uldhånds- krabbe), 3) Hemigrapsus sanguineus (stribet klippekrabbe), 4) Hemigrapsus takanoi (pensel-klippekrabbe), 5) Homarus ameri- canus (amerikansk hummer), 6) Paralithodes camtschaticus (Kamchatka-krabbe) and 7) Rhithropanopeus harrisii (østameri- kansk brakvandskrabbe). -

The World Lobster Market
GLOBEFISH RESEARCH PROGRAMME The world lobster market Volume 123 GRP123coverB5.indd 1 23/01/2017 15:06:37 FAO GLOBEFISH RESEARCH PROGRAMME VOL. 123 The world lobster market by Graciela Pereira Helga Josupeit FAO Consultants Products, Trade and Marketing Branch Fisheries and Aquaculture Policy and Resources Division Rome, Italy FOOD AND AGRICULTURE ORGANIZATION OF THE UNITED NATIONS Rome, 2017 The designations employed and the presentation of material in this information product do not imply the expression of any opinion whatsoever on the part of the Food and Agriculture Organization of the United Nations (FAO) concerning the legal or development status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. The mention of specific companies or products of manufacturers, whether or not these have been patented, does not imply that these have been endorsed or recommended by FAO in preference to others of a similar nature that are not mentioned. The views expressed in this information product are those of the author(s) and do not necessarily reflect the views or policies of FAO. ISBN 978-92-5-109631-4 © FAO, 2017 FAO encourages the use, reproduction and dissemination of material in this information product. Except where otherwise indicated, material may be copied, downloaded and printed for private study, research and teaching purposes, or for use in non-commercial products or services, provided that appropriate acknowledgement of FAO as the source and copyright holder is given and that FAO’s endorsement of users’ views, products or services is not implied in any way. -
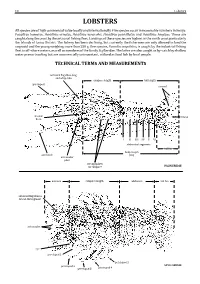
Lobsters LOBSTERS§
18 Lobsters LOBSTERS§ All species are of high commercial value locally and internationally. Five species occur in reasonable numbers in Kenya: Panulirus homarus, Panulirus ornatus, Panulirus versicolor, Panulirus penicillatus and Panulirus longipes. These are caughtungravid along and the the coast young by weighing the artisanal more fishing than 250 fleet. g. Landings One species, of these Puerulus species angulatus are highest in the north coast particularly the Islands of Lamu District. The fishery has been declining,Scyllaridae. but currently The latter the fishermen are also caught are only as by–catch allowed toby landshallow the , is caught by the industrial fishing fleet in off–shore waters, as well as members of the family water prawn trawling but areTECHNICAL commercially unimportant, TERMS AND utilized MEASUREMENTS as food fish by local people. and whip–like antennal flagellum long carapace length tail length pereiopod uropod frontal telson horn III III IV VIV abdominal segments tail fan body length antennule (BL) antennular plate strong spines on carapace PALINURIDAE antenna carapace length abdomen tail fan antennal flagellum a broad, flat segment antennules eye pereiopod 1 pereiopod 5 pereiopod 2 SCYLLARIDAE pereiopod 3 pereiopod 4 Guide to Families 19 GUIDE TO FAMILIES NEPHROPIDAE Page 20 True lobsters § To about 15 cm. Marine, mainly deep waters on soft included in the Guide to Species. 1st pair of substrates. Three species of interest to fisheriespereiopods are large 3rd pair of pereiopods with chela PALINURIDAE Page 21 Antennal Spiny lobsters § To about 50 cm. Marine, mostly shallow waters on flagellum coral and sand stone reefs, some species on soft included in the Guide to Species. -

Lobster Aquaculture Development in Vietnam and Indonesia 12
Lobster Aquaculture Development in Vietnam and Indonesia 12 Clive M. Jones, Tuan Le Anh, and Bayu Priyambodo Abstract Development of spiny (rock) lobster aquaculture is of special interest because mar- ket demand continues to increase while capture fisheries production remains static and with little likelihood of any increase. This chapter provides a synopsis of infor- mation about the history, development, status and future of tropical spiny lobster aquaculture with a particular focus on Vietnam and Indonesia, where considerable development has already occurred. Vietnam is the only country in the world where farming of lobsters is fully developed and commercially successful. The Vietnamese industry is based on a natural supply of seed lobsters – the puerulus stage, as hatch- ery supply is not yet available due to the difficult technical demands of rearing spiny lobster larvae in captivity. Vietnam currently produces around 1600 tonnes of premium grade lobsters, primarily of the species Panulirus ornatus, that are exported to China where the price is higher. The industry is valued at over $US120 million. This success led to significant interest in Indonesia where a fish- ery for seed lobsters has become well developed, with a catch 10–20 times greater than that of Vietnam. However, growout of lobster in Indonesia remains insignifi- cant due to adverse government policy and lack of farmer knowledge and skills. The seed lobsters available in Indonesia are primarily Panulirus homarus, a species with excellent production characteristics like P. ornatus, although with lesser value. Extraordinarily high abundance of naturally settling seed lobsters is appar- C. M. Jones (*) Centre for Sustainable Tropical Fisheries and Aquaculture, James Cook University, Cairns, QLD, Australia e-mail: [email protected] T. -

How to Become a Crab: Phenotypic Constraints on a Recurring Body Plan
Preprints (www.preprints.org) | NOT PEER-REVIEWED | Posted: 25 December 2020 doi:10.20944/preprints202012.0664.v1 How to become a crab: Phenotypic constraints on a recurring body plan Joanna M. Wolfe1*, Javier Luque1,2,3, Heather D. Bracken-Grissom4 1 Museum of Comparative Zoology and Department of Organismic & Evolutionary Biology, Harvard University, 26 Oxford St, Cambridge, MA 02138, USA 2 Smithsonian Tropical Research Institute, Balboa–Ancon, 0843–03092, Panama, Panama 3 Department of Earth and Planetary Sciences, Yale University, New Haven, CT 06520-8109, USA 4 Institute of Environment and Department of Biological Sciences, Florida International University, Biscayne Bay Campus, 3000 NE 151 Street, North Miami, FL 33181, USA * E-mail: [email protected] Summary: A fundamental question in biology is whether phenotypes can be predicted by ecological or genomic rules. For over 140 years, convergent evolution of the crab-like body plan (with a wide and flattened shape, and a bent abdomen) at least five times in decapod crustaceans has been known as ‘carcinization’. The repeated loss of this body plan has been identified as ‘decarcinization’. We offer phylogenetic strategies to include poorly known groups, and direct evidence from fossils, that will resolve the pattern of crab evolution and the degree of phenotypic variation within crabs. Proposed ecological advantages of the crab body are summarized into a hypothesis of phenotypic integration suggesting correlated evolution of the carapace shape and abdomen. Our premise provides fertile ground for future studies of the genomic and developmental basis, and the predictability, of the crab-like body form. Keywords: Crustacea, Anomura, Brachyura, Carcinization, Phylogeny, Convergent evolution, Morphological integration 1 © 2020 by the author(s). -

Prospects of Red King Crab Hepatopancreas Processing: Fundamental and Applied Biochemistry
Preprints (www.preprints.org) | NOT PEER-REVIEWED | Posted: 12 September 2020 doi:10.20944/preprints202009.0263.v1 Article Prospects of Red King Crab Hepatopancreas Processing: Fundamental and Applied Biochemistry Tatyana Ponomareva 1, Maria Timchenko 1, Michael Filippov 1, Sergey Lapaev 1, and Evgeny Sogorin 1,* 1 Federal Research Center "Pushchino Scientific Center for Biological Research of the RAS", Pushchino, Russia * Correspondence: [email protected]; Tel.: +7-915-132-54-19 Abstract: Since the early 1980s, a large number of research works on enzymes from the red king crab hepatopancreas have been conducted. These studies have been relevant both from a fundamental point of view for studying the enzymes of marine organisms and in terms of the rational management of nature to obtain new and valuable products from the processing of crab fishing waste. Most of these works were performed by Russian scientists due to the area and amount of waste of red king crab processing in Russia (or the Soviet Union). However, the close phylogenetic kinship and the similar ecological niches of commercial crab species and the production scale of the catch provide the bases for the successful transfer of experience in the processing of red king crab hepatopancreas to other commercial crab species mined worldwide. This review describes the value of recycled commercial crab species, discusses processing problems, and suggests possible solutions to these problems. The main emphasis is placed on the enzymes of the hepatopancreas as the most highly salubrious product of waste processed from red king crab fishing. Keywords: marine fisheries; aquatic organisms; brachyura; anomura; commercial crab species; red king crab; Kamchatka crab; processing waste; hepatopancreas; waste recycling; enzymes; proteases; hyaluronidase 1. -

Crustaceans Topics in Biodiversity
Topics in Biodiversity The Encyclopedia of Life is an unprecedented effort to gather scientific knowledge about all life on earth- multimedia, information, facts, and more. Learn more at eol.org. Crustaceans Authors: Simone Nunes Brandão, Zoologisches Museum Hamburg Jen Hammock, National Museum of Natural History, Smithsonian Institution Frank Ferrari, National Museum of Natural History, Smithsonian Institution Photo credit: Blue Crab (Callinectes sapidus) by Jeremy Thorpe, Flickr: EOL Images. CC BY-NC-SA Defining the crustacean The Latin root, crustaceus, "having a crust or shell," really doesn’t entirely narrow it down to crustaceans. They belong to the phylum Arthropoda, as do insects, arachnids, and many other groups; all arthropods have hard exoskeletons or shells, segmented bodies, and jointed limbs. Crustaceans are usually distinguishable from the other arthropods in several important ways, chiefly: Biramous appendages. Most crustaceans have appendages or limbs that are split into two, usually segmented, branches. Both branches originate on the same proximal segment. Larvae. Early in development, most crustaceans go through a series of larval stages, the first being the nauplius larva, in which only a few limbs are present, near the front on the body; crustaceans add their more posterior limbs as they grow and develop further. The nauplius larva is unique to Crustacea. Eyes. The early larval stages of crustaceans have a single, simple, median eye composed of three similar, closely opposed parts. This larval eye, or “naupliar eye,” often disappears later in development, but on some crustaceans (e.g., the branchiopod Triops) it is retained even after the adult compound eyes have developed. In all copepod crustaceans, this larval eye is retained throughout their development as the 1 only eye, although the three similar parts may separate and each become associated with their own cuticular lens. -

The Coconut Crab the Australian Centre for International Agricultural Research (ACIAR) Was Established in June 1982 by an Act of the Australian Parliament
The Coconut Crab The Australian Centre for International Agricultural Research (ACIAR) was established in June 1982 by an Act of the Australian Parliament. Its mandate is to help identify agricultural problems in developing countries and to commission collaborative research between Australian and developing country researchers in fields where Australia has a special research competence. Where trade names are used this constitutes neither endorsement of nor discrimination against any product by the Centre. ACIAR Monograph Series This peer-reviewed series contains the results of original research supported by ACIAR, or material deemed relevant to ACIAR's research objectives. The series is distributed internationally, with an emphasis on developing countries. Reprinted 1992 © Australian Centre for International Agricultural Research G.P.O. Box 1571, Canberra, ACT, Australia 2601 Brown, I.W. and Fielder, D .R. 1991. The Coconut Crab: aspects of the biology and ecology of Birgus Zatro in the Republic of Vanuatu. ACIAR Monograph No.8, 136 p. ISBN I 86320 054 I Technical editing: Apword Partners, Canberra Production management: Peter Lynch Design and production: BPD Graphic Associates, Canberra, ACT Printed by: Goanna Print, Fyshwick The Coconut Crab: aspects of the biology and ecology of Birgus latro in the Republic of Vanuatu Editors I.w. Brown and D.R. Fielder Australian Centre for International Agricultural Research, Canberra, Australia 199 1 The Authors I.W. Brown. Queensland Department of Primary Industries, Southern Fisheries Centre, PO Box 76, Deception Bay, Queensland, Australia D.R. Fielder. Department of Zoology, University of Queensland, St Lucia, Queensland, Australia W.J. Fletcher. Western Australian Marine Research Laboratories, PO Box 20, North Beach, Western Australia, Australia S. -

Distribution, Abundance, and Diversity of Epifaunal Benthic Organisms in Alitak and Ugak Bays, Kodiak Island, Alaska
DISTRIBUTION, ABUNDANCE, AND DIVERSITY OF EPIFAUNAL BENTHIC ORGANISMS IN ALITAK AND UGAK BAYS, KODIAK ISLAND, ALASKA by Howard M. Feder and Stephen C. Jewett Institute of Marine Science University of Alaska Fairbanks, Alaska 99701 Final Report Outer Continental Shelf Environmental Assessment Program Research Unit 517 October 1977 279 We thank the following for assistance during this study: the crew of the MV Big Valley; Pete Jackson and James Blackburn of the Alaska Department of Fish and Game, Kodiak, for their assistance in a cooperative benthic trawl study; and University of Alaska Institute of Marine Science personnel Rosemary Hobson for assistance in data processing, Max Hoberg for shipboard assistance, and Nora Foster for taxonomic assistance. This study was funded by the Bureau of Land Management, Department of the Interior, through an interagency agreement with the National Oceanic and Atmospheric Administration, Department of Commerce, as part of the Alaska Outer Continental Shelf Environment Assessment Program (OCSEAP). SUMMARY OF OBJECTIVES, CONCLUSIONS, AND IMPLICATIONS WITH RESPECT TO OCS OIL AND GAS DEVELOPMENT Little is known about the biology of the invertebrate components of the shallow, nearshore benthos of the bays of Kodiak Island, and yet these components may be the ones most significantly affected by the impact of oil derived from offshore petroleum operations. Baseline information on species composition is essential before industrial activities take place in waters adjacent to Kodiak Island. It was the intent of this investigation to collect information on the composition, distribution, and biology of the epifaunal invertebrate components of two bays of Kodiak Island. The specific objectives of this study were: 1) A qualitative inventory of dominant benthic invertebrate epifaunal species within two study sites (Alitak and Ugak bays). -

A Case Study of the Coconut Crab Birgus Latro on Zanzibar Highlights Global Threats and Conservation Solutions
A case study of the coconut crab Birgus latro on Zanzibar highlights global threats and conservation solutions T IM C ARO,HAJI H AMAD,RASHID S ULEIMAN R ASHID,ULRIKE K LOIBER V ICTORIA M. MORGAN,OSSI N OKELAINEN,BARNABAS C ARO,ILARIA P RETELLI N EIL C UMBERLIDGE and M ONIQUE B ORGERHOFF M ULDER Abstract The coconut crab Birgus latro, the largest terrestrial through local communities capitalizing on tourist revenue, decapod, is under threat in most parts of its geographical a conservation solution that could be applied more generally range. Its life cycle involves two biomes (restricted terrestrial across the species’ range. habitats near the coast, and salt water currents of the tropi- Keywords Birgus latro, coconut crab, conservation recom- cal Indian and Pacific Oceans). Its dependence on coastal mendations, IUCN, Pemba, population size, Tanzania habitat means it is highly vulnerable to the habitat destruc- tion that typically accompanies human population expan- sion along coastlines. Additionally, it has a slow reproductive rate and can reach large adult body sizes that, together with Introduction its slow movement when on land, make it highly susceptible to overharvesting. We studied the distribution and popula- he current wave of species extinctions in the tropics tion changes of coconut crabs at island sites in coastal Tis being driven by habitat loss and human exploitation. Tanzania on the western edge of the species’ geographical Species that are particularly susceptible to habitat conver- range. Our aim was to provide the data required for reassess- sion include those that depend on more than one biome ment of the extinction risk status of this species, which, despite to complete their life cycle, rendering them vulnerable to a indications of sharp declines in many places, is currently ca- wide variety of habitat changes or losses. -

Statewide Survey of Boat-Based Recreational Fishing in Western Australia 2015/16 K.L
Department of Primary Industries and Regional Development Fisheries Research Report No. 287 Statewide survey of boat-based recreational fishing in Western Australia 2015/16 K.L. Ryan, N.G. Hall, E.K. Lai, C.B. Smallwood, S.M. Taylor, B.S. Wise Fisheries Research Report No. 287 December 2017 Correct citation: Ryan KL, Hall NG, Lai EK, Smallwood CB, Taylor SM, Wise BS 2017. Statewide survey of boat- based recreational fishing in Western Australia 2015/16. Fisheries Research Report No. 287, Department of Primary Industries and Regional Development, Western Australia. 205pp. Enquiries: WA Fisheries and Marine Research Laboratories, PO Box 20, North Beach, WA 6920 Tel: +61 8 9203 0111 Email: [email protected] Website: www.fish.wa.gov.au A complete list of Fisheries Research Reports is available online at www.fish.wa.gov.au Important disclaimer The Chief Executive Officer of the Department of Primary Industries and Regional Development and the State of Western Australia accept no liability whatsoever by reason of negligence or otherwise arising from the use or release of this information or any part of it. Department of Primary Industries and Regional Development Gordon Stephenson House 140 William Street PERTH WA 6000 Telephone: (08) 6551 4444 Website: dpird.wa.gov.au ABN: 18 951 343 745 ISSN: 1035-4549 (Print) ISBN: 978-1-921258-00-8 (Print) ISSN: 2202-5758 (Online) ISBN: 978-1-921258-01-5 (Online) Copyright © Department of Primary Industries and Regional Development, 2017. 4874/17 ii Fisheries Research Report [Western Australia] No. 287 Table of Contents Executive Summary ............................................................................................... ix 1 Introduction .................................................................................................... -

(Paralithodes Camtschaticus) and Tanner Crab (Chionoecetes Bairdi) Growth, Condition, Calcification, and Survival
Effects of Ocean Acidification on Juvenile Red King Crab (Paralithodes camtschaticus) and Tanner Crab (Chionoecetes bairdi) Growth, Condition, Calcification, and Survival William Christopher Long*, Katherine M. Swiney, Caitlin Harris, Heather N. Page¤, Robert J. Foy Kodiak Laboratory, Resource Assessment and Conservation Engineering Division, Alaska Fisheries Science Center, National Marine Fisheries Service, NOAA, Kodiak, Alaska, United States of America Abstract Ocean acidification, a decrease in the pH in marine waters associated with rising atmospheric CO2 levels, is a serious threat to marine ecosystems. In this paper, we determine the effects of long-term exposure to near-future levels of ocean acidification on the growth, condition, calcification, and survival of juvenile red king crabs, Paralithodes camtschaticus, and Tanner crabs, Chionoecetes bairdi. Juveniles were reared in individual containers for nearly 200 days in flowing control (pH 8.0), pH 7.8, and pH 7.5 seawater at ambient temperatures (range 4.4–11.9 uC). In both species, survival decreased with pH, with 100% mortality of red king crabs occurring after 95 days in pH 7.5 water. Though the morphology of neither species was affected by acidification, both species grew slower in acidified water. At the end of the experiment, calcium concentration was measured in each crab and the dry mass and condition index of each crab were determined. Ocean acidification did not affect the calcium content of red king crab but did decrease the condition index, while it had the opposite effect on Tanner crabs, decreasing calcium content but leaving the condition index unchanged. This suggests that red king crab may be able to maintain calcification rates, but at a high energetic cost.