Vulvar Disease
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Reference Sheet 1
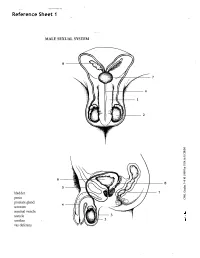
MALE SEXUAL SYSTEM 8 7 8 OJ 7 .£l"00\.....• ;:; ::>0\~ <Il '"~IQ)I"->. ~cru::>s ~ 6 5 bladder penis prostate gland 4 scrotum seminal vesicle testicle urethra vas deferens FEMALE SEXUAL SYSTEM 2 1 8 " \ 5 ... - ... j 4 labia \ ""\ bladderFallopian"k. "'"f"";".'''¥'&.tube\'WIT / I cervixt r r' \ \ clitorisurethrauterus 7 \ ~~ ;~f4f~ ~:iJ 3 ovaryvagina / ~ 2 / \ \\"- 9 6 adapted from F.L.A.S.H. Reproductive System Reference Sheet 3: GLOSSARY Anus – The opening in the buttocks from which bowel movements come when a person goes to the bathroom. It is part of the digestive system; it gets rid of body wastes. Buttocks – The medical word for a person’s “bottom” or “rear end.” Cervix – The opening of the uterus into the vagina. Circumcision – An operation to remove the foreskin from the penis. Cowper’s Glands – Glands on either side of the urethra that make a discharge which lines the urethra when a man gets an erection, making it less acid-like to protect the sperm. Clitoris – The part of the female genitals that’s full of nerves and becomes erect. It has a glans and a shaft like the penis, but only its glans is on the out side of the body, and it’s much smaller. Discharge – Liquid. Urine and semen are kinds of discharge, but the word is usually used to describe either the normal wetness of the vagina or the abnormal wetness that may come from an infection in the penis or vagina. Duct – Tube, the fallopian tubes may be called oviducts, because they are the path for an ovum. -

3 Embryology and Development
BIOL 6505 − INTRODUCTION TO FETAL MEDICINE 3. EMBRYOLOGY AND DEVELOPMENT Arlet G. Kurkchubasche, M.D. INTRODUCTION Embryology – the field of study that pertains to the developing organism/human Basic embryology –usually taught in the chronologic sequence of events. These events are the basis for understanding the congenital anomalies that we encounter in the fetus, and help explain the relationships to other organ system concerns. Below is a synopsis of some of the critical steps in embryogenesis from the anatomic rather than molecular basis. These concepts will be more intuitive and evident in conjunction with diagrams and animated sequences. This text is a synopsis of material provided in Langman’s Medical Embryology, 9th ed. First week – ovulation to fertilization to implantation Fertilization restores 1) the diploid number of chromosomes, 2) determines the chromosomal sex and 3) initiates cleavage. Cleavage of the fertilized ovum results in mitotic divisions generating blastomeres that form a 16-cell morula. The dense morula develops a central cavity and now forms the blastocyst, which restructures into 2 components. The inner cell mass forms the embryoblast and outer cell mass the trophoblast. Consequences for fetal management: Variances in cleavage, i.e. splitting of the zygote at various stages/locations - leads to monozygotic twinning with various relationships of the fetal membranes. Cleavage at later weeks will lead to conjoined twinning. Second week: the week of twos – marked by bilaminar germ disc formation. Commences with blastocyst partially embedded in endometrial stroma Trophoblast forms – 1) cytotrophoblast – mitotic cells that coalesce to form 2) syncytiotrophoblast – erodes into maternal tissues, forms lacunae which are critical to development of the uteroplacental circulation. -

Te2, Part Iii
TERMINOLOGIA EMBRYOLOGICA Second Edition International Embryological Terminology FIPAT The Federative International Programme for Anatomical Terminology A programme of the International Federation of Associations of Anatomists (IFAA) TE2, PART III Contents Caput V: Organogenesis Chapter 5: Organogenesis (continued) Systema respiratorium Respiratory system Systema urinarium Urinary system Systemata genitalia Genital systems Coeloma Coelom Glandulae endocrinae Endocrine glands Systema cardiovasculare Cardiovascular system Systema lymphoideum Lymphoid system Bibliographic Reference Citation: FIPAT. Terminologia Embryologica. 2nd ed. FIPAT.library.dal.ca. Federative International Programme for Anatomical Terminology, February 2017 Published pending approval by the General Assembly at the next Congress of IFAA (2019) Creative Commons License: The publication of Terminologia Embryologica is under a Creative Commons Attribution-NoDerivatives 4.0 International (CC BY-ND 4.0) license The individual terms in this terminology are within the public domain. Statements about terms being part of this international standard terminology should use the above bibliographic reference to cite this terminology. The unaltered PDF files of this terminology may be freely copied and distributed by users. IFAA member societies are authorized to publish translations of this terminology. Authors of other works that might be considered derivative should write to the Chair of FIPAT for permission to publish a derivative work. Caput V: ORGANOGENESIS Chapter 5: ORGANOGENESIS -

Management of Dental Trauma in a Primary Care Setting Abstract
Guidance for the Clinician in Rendering Pediatric Care CLINICAL REPORT Management of Dental Trauma in a Primary Care Setting Martha Ann Keels, DDS, PhD, and THE SECTION ON ORAL abstract HEALTH The American Academy of Pediatrics and its Section on Oral Health have KEY WORDS developed this clinical report for pediatricians and primary care physi- dental trauma, dental injury, tooth, teeth, dentist, pediatrician cians regarding the diagnosis, evaluation, and management of dental ABBREVIATION trauma in children aged 1 to 21 years. This report was developed CT—computed tomography through a comprehensive search and analysis of the medical and den- This document is copyrighted and is property of the American tal literature and expert consensus. Guidelines published and updated Academy of Pediatrics and its Board of Directors. All authors have filed conflict of interest statements with the American by the International Association of Dental Traumatology (www.dental- Academy of Pediatrics. Any conflicts have been resolved through traumaguide.com) are an excellent resource for both dental and non- a process approved by the Board of Directors. The American dental health care providers. Pediatrics 2014;133:e466–e476 Academy of Pediatrics has neither solicited nor accepted any commercial involvement in the development of the content of this publication. The guidance in this report does not indicate an exclusive INTRODUCTION course of treatment or serve as a standard of medical care. Variations, taking into account individual circumstances, may be By 14 years of age, 30% of children have experienced a dental injury.1 appropriate. Many of these children are taken directly to their medical home, an urgent care center, or an emergency department for evaluation and treatment. -

WO 2014/134709 Al 12 September 2014 (12.09.2014) P O P C T
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date WO 2014/134709 Al 12 September 2014 (12.09.2014) P O P C T (51) International Patent Classification: (81) Designated States (unless otherwise indicated, for every A61K 31/05 (2006.01) A61P 31/02 (2006.01) kind of national protection available): AE, AG, AL, AM, AO, AT, AU, AZ, BA, BB, BG, BH, BN, BR, BW, BY, (21) International Application Number: BZ, CA, CH, CL, CN, CO, CR, CU, CZ, DE, DK, DM, PCT/CA20 14/000 174 DO, DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, (22) International Filing Date: HN, HR, HU, ID, IL, IN, IR, IS, JP, KE, KG, KN, KP, KR, 4 March 2014 (04.03.2014) KZ, LA, LC, LK, LR, LS, LT, LU, LY, MA, MD, ME, MG, MK, MN, MW, MX, MY, MZ, NA, NG, NI, NO, NZ, (25) Filing Language: English OM, PA, PE, PG, PH, PL, PT, QA, RO, RS, RU, RW, SA, (26) Publication Language: English SC, SD, SE, SG, SK, SL, SM, ST, SV, SY, TH, TJ, TM, TN, TR, TT, TZ, UA, UG, US, UZ, VC, VN, ZA, ZM, (30) Priority Data: ZW. 13/790,91 1 8 March 2013 (08.03.2013) US (84) Designated States (unless otherwise indicated, for every (71) Applicant: LABORATOIRE M2 [CA/CA]; 4005-A, rue kind of regional protection available): ARIPO (BW, GH, de la Garlock, Sherbrooke, Quebec J1L 1W9 (CA). GM, KE, LR, LS, MW, MZ, NA, RW, SD, SL, SZ, TZ, UG, ZM, ZW), Eurasian (AM, AZ, BY, KG, KZ, RU, TJ, (72) Inventors: LEMIRE, Gaetan; 6505, rue de la fougere, TM), European (AL, AT, BE, BG, CH, CY, CZ, DE, DK, Sherbrooke, Quebec JIN 3W3 (CA). -

Preparation for Your Sex Life
1 Preparation for Your Sex Life Will every woman bleed during her first sexual intercourse? • Absolutely not. Not every woman has obvious bleeding after her first sexual intercourse. • Bleeding is due to hymen breaking during penetration of the penis into the vagina. We usually refer it as "spotting". It is normal and generally resolves. • Some girls may not have hymen at birth, or the hymen may have been broken already when engaging in vigorous sports. Therefore, there may be no bleeding. Is it true that all women will experience intolerable pain during their first sexual intercourse? • It varies among individuals. Only a small proportion of women report intolerable pain during their first sexual intercourse. The remaining report mild pain, tolerable pain or painless feeling. • Applying lubricants to genitals may relieve the discomfort associated with sexual intercourse; however, if you experience intolerable pain or have heavy or persistent bleeding during or after sexual intercourse, please seek medical advice promptly. How to avoid having menses during honeymoon? • To prepare in advance, taking hormonal pills like oral contraceptive pills or progestogens under doctor's guidance can control menstrual cycle and thereby avoid having menses during honeymoon. Is it true that women will get Honeymoon Cystitis easily during honeymoon? • During sexual intercourse, bacteria around perineum and anus may move upward to the bladder causing cystitis. Symptoms include frequent urination, difficulty and pain when urinating. • There may be more frequent sexual activity during honeymoon period, and so is the chance of having cystitis. The condition is therefore known as "honeymoon cystitis". • Preventive measures include perineal hygiene, drinking plenty of water, empty your bladder after sexual intercourse and avoid the habit of withholding urine. -

Saving Smiles Avulsion Pathway (Page 20) Saving Smiles: Fractures and Displacements (Page 22)
Greater Manchester Local Dental Network SavingSmiles Improving outcomes following dental trauma First Edition I Spring 2017 Practitioners’ Toolkit Contents 04 Introduction to the toolkit from the GM Trauma Network 06 History & examination 10 Maxillo-facial considerations 12 Classification of dento-alveolar injuries 16 The paediatric patient 18 Splinting 20 The AVULSED Tooth 22 The BROKEN Tooth 23 Managing injuries with delayed presentation SavingSmiles 24 Follow up Improving outcomes 26 Long term consequences following dental trauma 28 Armamentarium 29 When to refer 30 Non-accidental injury 31 What should I do if I suspect dental neglect or abuse? 34 www.dentaltrauma.co.uk 35 Additional reference material 36 Dental trauma history sheet 38 Avulsion pathways 39 Fractues and displacement pathway 40 Fractures and displacements in the primary dentition 41 Acknowledgements SavingSmiles Improving outcomes following dental trauma Ambition for Greater Manchester Introduction to the Toolkit from The GM Trauma Network wish to work with our colleagues to ensure that: the GM Trauma Network • All clinicians in GM have the confidence and knowledge to provide a timely and effective first line response to dental trauma. • All clinicians are aware of the need for close monitoring of patients following trauma, and when to refer. The Greater Manchester Local Dental Network (GM LDN) has established a ‘Trauma Network’ sub-group. The • All settings have the equipment described within the ‘armamentarium’ section of this booklet to support optimal treatment. Trauma Network was established to support a safer, faster, better first response to dental trauma and follow up care across GM. The group includes members representing general dental practitioners, commissioners, To support GM practitioners in achieving this ambition, we will be working with Health Education England to provide training days and specialists in restorative and paediatric dentistry, and dental public health. -

Localised Provoked Vestibulodynia (Vulvodynia): Assessment and Management
FOCUS Localised provoked vestibulodynia (vulvodynia): assessment and management Helen Henzell, Karen Berzins Background hronic vulvar pain (pain lasting more than 3–6 months, but often years) is common. It is estimated to affect 4–8% of Vulvodynia is a chronic vulvar pain condition. Localised C women at any one time and 10–20% in their lifetime.1–3 provoked vestibulodynia (LPV) is the most common subset Little attention has been paid to the teaching of this condition of vulvodynia, the hallmark symptom being pain on vaginal so medical practitioners may not recognise the symptoms, and penetration. Young women are predominantly affected. LPV diagnosis is often delayed.2 Community awareness is low, but is a hidden condition that often results in distress and shame, increasing with media attention. Women can be confused by the is frequently unrecognised, and women usually see a number symptoms and not know how to discuss vulvar pain. The onus is of health professionals before being diagnosed, which adds to on medical practitioners to enquire about vulvar pain, particularly their distress and confusion. pain with sex, when taking a sexual or reproductive health history. Objective Vulvodynia The aim of this article is to inform health providers about the Vulvodynia is defined by the International Society for the Study assessment and management of LPV. of Vulvovaginal Disease (ISSVD) as ‘chronic vulvar discomfort, most often described as burning pain, occurring in the absence Discussion of relevant findings or a specific, clinically identifiable, neurologic 4 Diagnosis is based on history. Examination is used to support disorder’. It is diagnosed when other causes of vulvar pain have the diagnosis. -

The Vulva Vaginal Diseases in Daily Practice Layout 1
Experimental & Clinical Article Gynecology; and Gynecologial Onncology The Vulva / Vaginal Diseases in Daily Practice Gamze S. ÇAĞLAR1, Elif D. ÖZDEMİR1, Sevim D. CENGİZ1, Handan DOĞAN2 Ankara, Turkey OBJECTIVE: To emphasize the neglected vulva/vaginal lesions and symptoms commonly encountered in daily practice. STUDY DESIGN: The data of 98 patients with vulva or vaginal biopsies were collected retrospectively. The histopathological diagnosis of 82(83.67%) vulvar and 16(16.32%) vaginal biopsy cases were eval- uated. RESULTS: The most common symptom was mass in 67% and 62% of cases in vulvar and vaginal re- gion, respectively. Among the vulvar lesions the most frequent histopathological diagnoses were condy- loma acuminatum (20.73%), hyperkeratotic papilloma (14.63%), non-spesific inflammatory changes (12.19%), fibroepithelial polyp (9.75%) and bartholin cyst (8.53%). On the other hand, the histopatolog- ical evaluation of the vaginal lesions revealed vaginal stromal polyp (25%) and gartner cyst (25%) as the most frequent lesions. CONCLUSIONS: The results of the study document lesions commonly not well known or disregarded by gynecologists. The literature is inconclusive about vulvavaginal diseases. Expanded data will help the clinicians in making correct diagnoses and patient managament. Key Words: Fibroepithelial polyp, Hyperkeratotic papilloma, Stromal polyp, Vaginal diseases, Vulvar diseases. Gynecol Obstet Reprod Med 2011;17:155-159 Introduction The clinicians should be aware of these physiological changes that will help in guiding the vulvar/vaginal symptoms Vulva is once called forgetten pelvic organ and related and disorders in daily clinical practice. There is limited data symptoms are usually considered as unimportant.1 The most about the most common reported histopathological diagnoses common compliants in women admitting to gynecology clin- and management in vulvavaginal diseases. -

Impact of a Multidisciplinary Vulvodynia Program on Sexual Functioning and Dyspareunia
238 Impact of a Multidisciplinary Vulvodynia Program on Sexual Functioning and Dyspareunia Lori A. Brotto, PhD, Paul Yong, MD, Kelly B. Smith, PhD, and Leslie A. Sadownik, MD Department of Gynaecology, University of British Columbia, Vancouver, BC, Canada DOI: 10.1111/jsm.12718 ABSTRACT Introduction. For many years, multidisciplinary approaches, which integrate psychological, physical, and medical treatments, have been shown to be effective for the treatment of chronic pain. To date, there has been anecdotal support, but little empirical data, to justify the application of this multidisciplinary approach toward the treatment of chronic sexual pain secondary to provoked vestibulodynia (PVD). Aim. This study aimed to evaluate a 10-week hospital-based treatment (multidisciplinary vulvodynia program [MVP]) integrating psychological skills training, pelvic floor physiotherapy, and medical management on the primary outcomes of dyspareunia and sexual functioning, including distress. Method. A total of 132 women with a diagnosis of PVD provided baseline data and agreed to participate in the MVP. Of this group, n = 116 (mean age 28.4 years, standard deviation 7.1) provided complete data at the post-MVP assessment, and 84 women had complete data through to the 3- to 4-month follow-up period. Results. There were high levels of avoidance of intimacy (38.1%) and activities that elicited sexual arousal (40.7%), with many women (50.4%) choosing to focus on their partner’s sexual arousal and satisfaction at baseline. With treatment, over half the sample (53.8%) reported significant improvements in dyspareunia. Following the MVP, there were strong significant effects for the reduction in dyspareunia (P = 0.001) and sex-related distress (P < 0.001), and improvements in sexual arousal (P < 0.001) and overall sexual functioning (P = 0.001). -

Japan Society of Gynecologic Oncology Guidelines 2015 for the Treatment of Vulvar Cancer and Vaginal Cancer
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Tsukuba Repository Japan Society of Gynecologic Oncology guidelines 2015 for the treatment of vulvar cancer and vaginal cancer 著者 Saito Toshiaki, Tabata Tsutomu, Ikushima Hitoshi, Yanai Hiroyuki, Tashiro Hironori, Niikura Hitoshi, Minaguchi Takeo, Muramatsu Toshinari, Baba Tsukasa, Yamagami Wataru, Ariyoshi Kazuya, Ushijima Kimio, Mikami Mikio, Nagase Satoru, Kaneuchi Masanori, Yaegashi Nobuo, Udagawa Yasuhiro, Katabuchi Hidetaka journal or International Journal of Clinical Oncology publication title volume 23 number 2 page range 201-234 year 2018-04 権利 (C) The Author(s) 2017. This article is an open access publication This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons. org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. URL http://hdl.handle.net/2241/00151712 doi: 10.1007/s10147-017-1193-z Creative Commons : 表示 http://creativecommons.org/licenses/by/3.0/deed.ja Int J Clin Oncol (2018) 23:201–234 https://doi.org/10.1007/s10147-017-1193-z SPECIAL ARTICLE Japan Society of Gynecologic Oncology guidelines 2015 for the treatment of vulvar cancer and vaginal cancer Toshiaki Saito1 · Tsutomu Tabata2 · Hitoshi Ikushima3 · Hiroyuki Yanai4 · Hironori Tashiro5 · Hitoshi Niikura6 · Takeo Minaguchi7 · Toshinari Muramatsu8 · Tsukasa Baba9 · Wataru Yamagami10 · Kazuya Ariyoshi1 · Kimio Ushijima11 · Mikio Mikami8 · Satoru Nagase12 · Masanori Kaneuchi13 · Nobuo Yaegashi6 · Yasuhiro Udagawa14 · Hidetaka Katabuchi5 Received: 29 August 2017 / Accepted: 5 September 2017 / Published online: 20 November 2017 © The Author(s) 2017. -

Ovarian Insufficiency and CTNNB1 Mutations Drive Malignant Transformation of Endometrial Hyperplasia with Altered PTEN/PI3K Activities
Ovarian insufficiency and CTNNB1 mutations drive malignant transformation of endometrial hyperplasia with altered PTEN/PI3K activities Jumpei Terakawaa,b,1,2, Vanida Ann Sernaa,b,1, Makoto Mark Taketoc, Takiko Daikokud, Adrian A. Suarezb,e, and Takeshi Kuritaa,b,3 aDepartment of Cancer Biology and Genetics, Ohio State University, Columbus, OH 43210; bThe Comprehensive Cancer Center, Ohio State University, Columbus, OH 43210; cDivision of Experimental Therapeutics, Graduate School of Medicine, Kyoto University, Yoshida-Konoe-cho, Sakyo-ku, 606-8506 Kyoto, Japan; dDivision of Transgenic Animal Science, Advanced Science Research Center, Kanazawa University, 920-8640 Kanazawa, Japan; and eDepartment of Pathology, Ohio State University, Columbus, OH 43210 Edited by Patricia K. Donahoe, Pediatric Surgical Research Laboratories, Massachusetts General Hospital, Department of Surgery, Harvard Medical School, Boston, MA, and approved January 23, 2019 (received for review August 28, 2018) Endometrioid endometrial carcinomas (EECs) carry multiple driver PI3K (5). However, PTEN and PI3K (PIK3CA or PIK3R1)mu- mutations even when they are low grade. However, the biological tations co-occurred in 67% (59/88) of CL-EECs. This observation significance of these concurrent mutations is unknown. We explored questions the widely accepted concept that the loss of PTEN and the interactions among three signature EEC mutations: loss-of- activation of PI3K have synonymous effects on cellular physiology, as function (LOF) mutations in PTEN, gain-of-function (GOF) mutations they catalyze opposite reactions: PI3K converts phosphatidylinositol of phosphoinositide 3-kinase (PI3K), and CTNNB1 exon 3 mutations, (4,5)-biphosphate (PIP2) to phosphatidylinositol (3,4,5)-trisphosphate utilizing in vivo mutagenesis of the mouse uterine epithelium.