The Apothecary Newfoundland and Labrador Pharmacy Board Spring 2009
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

ANNUAL REPORT 2009 - 2010 from the Director
School of Pharmacy ANNUAL REPORT 2009 - 2010 From the Director I am very pleased to present Memorial University’s School of Pharmacy 2009- 2010 annual report. This report provides an overview of how our program is affecting the future of health care in Newfoundland and Labrador and shows our appreciation to our alumni, friends and corporate partners who make this possible. For over 20 years, the School has been growing as a place of innovation, creativity and excellence in pharmacy education. Since 1989, we have graduated 702 students. We are proud to know that they now work in every corner of our province and country, as well as around the world, in all aspects of pharmaceutical practice and research. Our need to continue this growth is spurred by a regional urgency for more pharmacists in our health care system. During the past year we have been engaged in a number of activities as we plan to increase student enrolment. The lack of physical facilities to accommodate larger classes and faculty and staff present unique challenges. In their 2009 review, the Canadian Council for Accreditation of Pharmacy Programs identified the School’s growth is at risk due to inadequate space and facilities. We will continue to work with government to address this challenge, expand the program and satisfy the requirement for more pharmacy graduates to help meet the health care needs of Newfoundlanders and Labradorians. I am extremely proud of the accomplishments of our faculty, staff and students and I am glad you are taking the time to review this report. -

Adverse Events Following Immunization Data Submission and Response Guidelines Adverse Events Following Immunization
Alberta Alberta Health Health Adverse Events Following Immunization Data Submission and Response Guidelines Adverse Events Following Immunization Data Submission and Response Guidelines Version 6.7 September 2018 Version 6.4 SupersededDecember 2015 Alberta Health Adverse Events to Immunization Data Submission Guidelines September 2018 Document Revisions Version Date Revision Description 6.7 September 2018 Submitter Prefix Codes(Table 2) • 33 - Workplace Health and Safety – AHS • 34 - Workplace Health and Safety – Covenant Health • 45 - Fort McKay First Nation • 46 – Alexis First Nation • 47 – Beaver First Nation • 48 – Lubicon Lake Nation Vaccine Codes (Table 33) Added Vaccine Codes: • Var-SI Shingles Inactivated • MenB FHbp Meningococcal B FHbp (recombinant) Changed description of Var-S vaccine Changed description of Vaccine Codes • Var-S Shingles Live • RSVIg Respiratory Syncytial Virus • Meningococcal B (4C, OMV recombinant, absorbed) Delivery Management Site Codes (Table 27) • 195 - Workplace Health and Safety – AHS • 196 - Workplace Health and Safety – Covenant Health • 320 - Safeway 8863 (St. Albert) • 321 - Safeway 8830 (Airdrie) • 322 - Safeway 8897 (Spruce Grove) • 323 - Safeway 8886 Callingwood (Edmonton) • 324 – Safeway 8912 Garrison Woods (Calgary) • 325 - Safeway 8877 Red Deer • 326 – Safeway 8894 (Fort Saskatchewan) • 327 – Safeway 8885 (Stoney Plain) • 328 - Safeway 8842 (Glenmore Landing) • 329 - Discovery Ridge Compounding Pharmacy & Travel Clinic • 856 - Fort McKay Health Centre • 857 – Alexis Health Services • 858 – -

Annual Report 2018
ANNUAL REPORT 2018 PATIENT FOCUSED PHARMACY PRACTICE 1 TABLE OF CONTENTS Message from the Chair and Registrar 1 About the Board 2 Meet the New Board Members 3 Board Members 4 Board Committees 5 Quality Assurance Program 7 Practice Site Assessments 8 Registrant Based Activities 9 Licensing and Registration 10 Addressing Practice Concerns 11 Professional Practice Development 12 Communications 13 Minutes of 2018 Annual General Meeting 14 2018 Summarized Financial Statements 16 MESSAGE FROM THE CHAIR AND REGISTRAR It is with great pleasure we present the In 2018, great strides were made in the 2018 Annual Report for the Newfoundland development of new and updated standards and Labrador Pharmacy Board (NLPB). We and policies to guide our registrants in had a productive year of activities in each optimization of patient-focused pharmacy of our four business lines: registration and care. In response to the increased licensing, quality assurance, professional government action taken to reduce and practice, and complaints and discipline. prevent opioid related harms we further enriched our direction, education and Throughout the year we conducted business resources for pharmacy professionals as usual keeping our core values top of mind on the importance of providing Opioid while working to advance pharmacy care Agonist Maintenance Treatment. Other new for a safe and healthy community. Our goals additions include an interpretation guide on and objectives are strategically developed Ending the Pharmacist-Patient Relationship, in advance of the year ahead so that we are and a practice policy on Registrant Use of accountable to the people of the province. Social Media. Our annual outlook is aligned to best ensure our registrants are providing safe and quality As the scope of pharmacy practice continues Colleen Squires, Board Chair pharmacy care in accordance with the to grow, the regulatory processes to ensure Pharmacy Act, 2012, its Regulations, By-Laws safe and quality practice increase also. -

Employee Handbook Barrington Passage Pharmacy Ltd
Employee Handbook Barrington Passage Pharmacy Ltd. Barrington Passage Pharmacy Ltd. Introduction: This Employee Handbook has been prepared as an information resource to confirm the policies and procedures that pertain to the normal business operation of Barrington Passage Pharmacy Ltd. and the philosophy and standards by which we operate. All employees must read and observe the policies and procedures described in this book. The Owners/ Management are committed to following these policies and procedures but reserve the right to take alternative steps if deemed necessary to manage the workplace. This is a working document and the Owner/Management reserve the right to change these policies at any time. Revisions to this Handbook will be done so in writing. Due to the nature of the Dispensary Operations, some policies and procedures may be different for Pharmacy staff and may be included in a separate document. All employees will be required to read the Handbook then sign the Acknowledgement Form upon initial receipt of this document to confirm that they have done so. At any time if you have any questions, concerns or comments, you should not hesitate to speak to your supervisor. Sincerely, Kim Geldart, PhC. Owner 2 Table of Contents Topic Page Last Updated Store Information Welcome to Pharmasave Workplace 4-11 January 2017 Policies and Procedure 12 January 2017 Probation Period 13 January 2017 Performance Review 14 January 2017 Pay Schedule 15 January 2017 Overtime Pay 16 January 2017 Work Schedules 17 January 2017 Holiday Pay 18 January -

PAM2021 Commstoolkit Final.Pdf
1 Contents About PAM 3 10 ways to get involved 4 Key messages 6 Email & newsletter template 7 #MyFavePharm Contest 8 Celebrate your pharmacy team 9 Social media posts 10 Graphics 14 Printable material 16 2 About PAM Each March we celebrate the contributions that pharmacy professionals make to our health-care system and help educate Canadians about the health-care services and advice their pharmacists are now delivering. This year, we’ve put a new spin on things. Pharmacy Awareness Month has moved from ‘awareness’ to ‘appreciation’ to better reflect and celebrate the extraordinary role pharmacy professionals play in health care, especially during the COVID-19 pandemic. Join us in celebrating Pharmacy Appreciation Month this March! This toolkit This toolkit includes a range of information and resources to help you celebrate PAM and communicate your activities to your stakeholders, patients and the public. National and provincial pharmacy associations, pharmacy students and pharmacy professionals from across Canada will be celebrating PAM 2021 throughout March. Join the conversation and use some of these resources to get started. Visit pharmacists.ca/pam for more resources and information. About CPhA The Canadian Pharmacists Association (CPhA) is the uniting national voice of pharmacy and the pharmacist profession in Canada. As pharmacists undertake an enhanced role in the delivery of health care services, CPhA ensures that the profession is recognized as a national leader in health care, influencing the policies, programs, budgets and initiatives affecting the profession and the health of Canadians. More information is available at www.pharmacists.ca. Follow us: @CPhAAPhC @CPhA @cpha_aphc CPhATV 3 10 ways to get involved 1. -

Rubicon Pharmacies Is Seeking a Store Operator/Pharmacy Manager
Rubicon Pharmacies is seeking a Store Operator/Pharmacy Manager to lead their team at Pharmasave #415 located in Biggar, SK! The store is open Monday to Friday from 8:30am to 7:00pm and Saturday from 9:00am to 5:00pm. This position will oversee the entire operation of Pharmasave #415. They will manage a staff of skilled pharmacists and pharmacy assistants. They will be a leader within the community and drive pharmacy services to better serve Biggar and its surrounding area. This position will ensure profitability through effective budgeting, inventory control and promotional activities. We offer competitive employment packages, including benefits & pension, and added incentives for certain markets. Rubicon strongly supports internal movement across and upward in our growing network. We believe in helping you achieve your career goals, while maintaining a healthy work-life balance at the same time. Rubicon is a growing company with over 100 locations throughout British Columbia, Alberta, Saskatchewan and Manitoba. Rubicon operates its stores within the Pharmasave, PharmaChoice, Value Drug Mart, Remedy’s RX and IDA franchise systems. We provide our teams with an extensive support network, and a variety of clinical programs, best-practices, and tools to succeed. Investing in innovations such as central-fill models and automated dispensing technology, allows local pharmacy teams more opportunity to focus on providing personalized, patient-centered care, while building adherence. Rubicon has embraced practice innovations and will continue developing programs and services to support our local pharmacy teams. JOB DESCRIPTION: STORE OPERATOR/PHARMACY MANAGER OBJECTIVE: This manager-level position is responsible for overseeing fulfillment functions to ensure the operation provides high quality care and customer service, and operates efficiently through optimisation of its human, physical, and financial capital within the framework of legislative requirements, and Rubicon policies and programs. -

CARDIOVASCULAR PROGRAM Participating Pharmacies in Ontario
PHARMACIST HEALTH COACHING - CARDIOVASCULAR PROGRAM Participating Pharmacies in Ontario Please check back regularly for updated information. Store Pharmacy Name Address City Province Postal Code Pharmacy Phone Number Remedy's Rx 441 401 Ottawa St. Almonte Ontario K0A 1A0 (613) 256-6884 Emrose Medical Pharmacy 290 Sandwich St. S. Amherstburg Ontario N9V 2A8 (519) 736-2223 Costco Pharmacy 1105 100 Legend Crt. Ancaster Ontario L9K 1J3 (905) 304-2451 Alliance Pharmacy Inc. 20 Furbacher Lane - Unit 2 Aurora Ontario L4G 6W1 (905) 713-3944 Shoppers Drug Mart 1464 380 Mapleview Dr. W. Barrie Ontario L4N 9N4 (705) 792-6244 Belle River Pharmasave 9728 520 Notre Dame St. Belle River Ontario N0R 1A0 (519) 715-9673 Bowmanville Clinic Pharmacy 222 King St. E. Bowmanville Ontario L1E 1C6 (905) 623-7611 Shoppers Drug Mart 1199 10048 Mclaughlin Rd. Brampton Ontario L7A 2X6 (905) 846-4444 Shoppers Drug Mart 1369 10970 Airport Rd Brampton Ontario L6R 0E1 (905) 793-0308 Remedy's Rx 380 222 Fairview Dr Brantford Ontario N3R 2W9 (519) 759-3784 Shoppers Drug Mart 1155 269 Clarence Street Brantford Ontario N3R 3T6 (519) 753-9091 Loblaw Pharmacy 4067 1059 Plains Rd E Burlington Ontario L7T 4K1 (905) 634-2739 Preston Medical Pharmacy 125 Waterloo St. S. Cambridge Ontario N3H 1N3 (519) 653-1870 Carleton Place IDA 47 Lansdowne Park Carleton Ontario K1N 0B7 (613) 257-1414 Pharmacy 101 464 Division Street Cobourg Ontario K9A 3S2 (905) 372-8808 Health Centre Pharmacy 34 1450 Hwy #2 Courtice Ontario L1E 3C3 (905) 721-0011 Shoppers Drug Mart 1056 133 King Street west Dundas Ontario L9H 1V3 (905) 628-6121 Centre Wellington Remedy's Rx 1-855 St. -
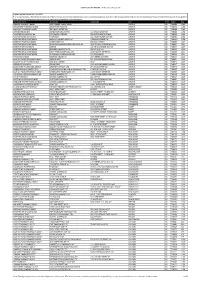
DISPENSING FEE REPORT - All Provinces Except ON
DISPENSING FEE REPORT - All Provinces Except ON Dispensing Fee Report Jan - Mar 2021 * In some provinces, a tiered fee schedule is in effect and thus some of the reported data may be skewed based upon such tiers. We strongly advise that you call the specific pharmacy of interest to ensure that you get the most updated, accurate fee information for the particular prescription that you require. Pharmacy Name Address(1) Address(2) City Prov. Postal Avg LOBLAW PHARMACY #4951 1050 YANKEE VALLEY ROAD AIRDRIE AB T4A2E4 11.48 DRUGSTORE PHARMACY #1540 300 AIRDRIE ROAD NE AIRDRIE AB T4B3P2 11.60 WALMART PHARMACY #1050 2881 MAIN STREET SW AIRDRIE AB T4B3G5 11.68 LONDON DRUGS #84 LONDON DRUGS LIMITED 110-2781 MAIN ST SW AIRDRIE AB T4B3S6 11.94 PHARMEDIC PHARMACY #4 FATIMA SERVICES INC 506-3 STONEGATE DR NW AIRDRIE AB T4B0N2 11.99 POLARIS TRAVEL CLINIC AND PHARMACY 404-191 EDWARDS WAY SW AIRDRIE AB T4B3E2 12.04 SHOPPERS DRUG MART #2429 NARAYAN INVESTMENTS INC 2-804 MAIN STREET SE AIRDRIE AB T4B3M1 12.06 SAFEWAY PHARMACY #8830 SOBEYS WEST INC. 505 MAIN STREET AIRDRIE AB T4B2B8 12.07 SHOPPERS DRUG MART #2431 GINCHER PHARMACEUTICAL SERVICES INC UNIT 801-401 COOPER BLVD SW AIRDRIE AB T4B4J3 12.07 SOBEYS PHARMACY #5172 AIRDRIE 100, 65 MACKENZIE WAY SW AIRDRIE AB T4B0V7 12.09 SHOPPERS DRUG MART #2360 SELINGER DRUGGISTS LTD. 836 1ST AVE NW AIRDRIE AB T4B2R3 12.10 SHOPPERS DRUG MART #2328 WOLF PHARMACEUTICALS LTD 1200 MARKET STREET S.E. AIRDRIE AB T4A0K9 12.11 SHOPPERS DRUG MART #2386 M. -

Pharmacy Name Pharmacy Address City/Town Province Postal Code RHA Harder's Pharmacy Box 549, 604 Herbert Ave
Pharmacy Name Pharmacy Address City/Town Province Postal Code RHA Harder's Pharmacy Box 549, 604 Herbert Ave. Herbert Saskatchewan S0H 2A0 Cypress Dirk Family Pharmacy Ltd 116-1st Ave. W. Box 400 Leader Saskatchewan S0N 1H0 Cypress Leader Pharmacy 100-1st Ave. W. Leader Saskatchewan S0N 1H0 Cypress Pharmasave #410 118 Jasper Street Maple Creek Saskatchewan S0N 1N0 Cypress Pharmasave #403 256B Centre Street, Box 148 Ponteix Saskatchewan S0N 1Z0 Cypress Jae's Pharmacy 335 Centre Street Shaunavon Saskatchewan S0N 2M0 Cypress Rx Drug Mart #3004 353 Center Street Shaunavon Saskatchewan S0N 2M0 Cypress Drugstore Pharmacy #6705 1501 North Service Road E. Swift Current Saskatchewan S9H 4X7 Cypress Pharmasave #406 390 Central Ave. N. Swift Current Saskatchewan S9H 0L4 Cypress Pioneer Co-op Drugs #4 1150 Central Ave. N. Swift Current Saskatchewan S9H 0G1 Cypress Rexall Drug Store #7301 780 Central Ave North Swift Current Saskatchewan S9H 0M3 Cypress Shoppers Drug Mart #2456 911 Central Ave. N. Swift Current Saskatchewan S9H 3V3 Cypress Wal-Mart Pharmacy #3099 1800-22nd Ave. N.E. Swift Current Saskatchewan S9H 0E5 Cypress Shoppers Drug Mart #433 424 Centre Street Assiniboia Saskatchewan S0H 0B0 Five Hills Southland Co-op Pharmacy 400 Centre Street, Box 490 Assiniboia Saskatchewan S0H 0B0 Five Hills Rexall #7317 511 Main Street, P.O. Box 720 Gravelbourg Saskatchewan S0H 1X0 Five Hills Alive & Well Health Consulting (LaFleche Pharmacy) Box 400, 119 Main Street LaFleche Saskatchewan S0H 2K0 Five Hills Loblaw Pharmacy 1583 30 Thatcher Dr. E. Moose Jaw Saskatchewan S6J 1L7 Five Hills Medicine Shoppe Pharmacy #293 323 4th Avenue SW Moose Jaw Saskatchewan S6H 5V2 Five Hills Moose Jaw Co-op Pharmacy 500A-1st Av. -

Scope Acquisition Update
1 BOARD OF DIRECTORS BIOS BOARD COMPOSITION Corix is governed by a public‐style Board of Directors. There are currently seven directors on the Board, which includes representatives from bcIMC, CAI and the Company management. Gordon R. Barefoot, Chairman Gordon Barefoot is a director of the Corporation and a CAI nominated representative to the Board. He has been the President of Cabgor Management Inc. since 2005. Previously, Gord was Senior Vice‐President Finance and Chief Financial Officer of Terasen (now FortisBC) and held other senior vice‐president roles from 1998 to 2005. Prior to that, he worked for Ernst and Young from 1979 to 1998 and became a partner of that firm in 1985. Gord is on the board of directors and is the chairman of the audit committee of Nventa Biopharmaceuticals Corporation, a TSX listed company. He is also Chairman of the board of directors and chairman of the audit committee for AutoCanada Inc. Gord received a Bachelor of Commerce with Honours from the University of Manitoba, is a Chartered Accountant and is a Certified Director and a member of the Institute of Corporate Directors. Brett Hodson Brett Hodson has worked in the energy and water utility industries for over 20 years, first with BC Hydro then joining Terasen (now FortisBC) in 1991. At Terasen, Brett held a number of positions in the areas of resource planning, marketing, regulatory pricing, and business development. For the six years prior to the Kinder Morgan acquisition, Brett had been the President of TWUS and was a member of the Executive Management Committee of Terasen. -

Survey of Canadian Community Pharmacists: Use of Digital Health Technologies in Practice August 2014
Final Report Survey of Canadian Community Pharmacists: Use of Digital Health Technologies in Practice August 2014 Commissioned by Background and Methodology . Canada Health Infoway, in partnership with the Canadian Pharmacists Association, invited community pharmacists to participate in a survey to understand their use and perceived benefits of electronic systems in practice. To this end, Harris/Decima was asked to host an online survey and analyze the results from it. The CPhA and provincial pharmacy associations distributed email invitations to its members. 447* surveys were completed . In addition, the data were weighted to reflect the universe of pharmacists in a community setting. Source document was the Pharmacists Workforce, 2012 from the CIHI. Weighting factors that were applied are well within the industry standards (under 2.5) *447 surveys were completed across 9 provinces as follow : AB (n=65), BC (n=55), MB (n=20), NB (n=21), NL (n=23), NS (n=25), ON (n=114), PEI (n=27), SK (n=97). Note that analysis by province is not provided in this presentation. Quebec, Nunavut , NWT and Yukon did not participate in this survey. Commissioned by 2 Respondent Profile Commissioned by : Respondents represent various community practice settings Q2. Please specify your main community pharmacy practice setting: Check only ONE Community Pharmacy – independently 24% owned 30% Community Pharmacy – chain/franchise (e.g. Shoppers Drug Mart, Jean Coutu, Pharma Plus, Lawtons) Community Pharmacy – pharmacy located within a large retail operation Community Pharmacy – banner (e.g. I.D.A, Guardian, Uniprix, Price Watchers, 27% Pharmasave) 18% Base: All respondents (n=447) Commissioned by 4 A majority process two hundred or fewer prescriptions Q3. -

Honouring Exceptional Women
V22N1 2011:V20N3 2009 12/13/10 11:22 AM Page 8 BRIEFS Honouring Exceptional Women 9 Atlantic Canadians receive WXN awards On a per capita basis, Atlantic Canada has a larger representation in the 2010 list of Canada’s Most Powerful Women: Top 100TM than any other region in the country. Eight of this year’s Top 100 winners, and one of the 11 Hall of Fame inductees, are from New Brunswick, Nova Scotia and Newfoundland and Labrador. According to the awards organizer, Women’s Executive Network (WXN), the awards pro- gram is Canada’s most recognizable award for the country’s highest achieving female leaders in the private, public and not-for-profit sectors. “They each have remarkable achievements and Top 100 is a mark of their success and how they have supported their communities, com- panies, and organizations. Our Top 100 community is a powerful group of women who are at the top of their game, exemplify success and empower the next generation of women to take The Honourable Kathy Dunderdale with the Lieutenant Governor, His Honour, the Honourable John C. Crosbie, their careers to the next level,” said Pamela Jeffery, founder, Women’s Executive Network. signs her Oath of Office as she becomes the 10th Premier The Awards cover eight categories: Sun Life Financial Arts & Communications, Canadian of Newfoundland and Labrador. (Don Lane photo) Breast Cancer Foundation Champions, Scotiabank Corporate Executives, Franklin Temple- ton Investments/Fiduciary Trust Entrepreneurs, TELUS Future Leaders, KPMG Professionals, Woman Premier Cisco Public Sector Leaders, and Xstrata Nickel Trailblazers & Trendsetters. For Newfoundland (TOP TO BOTTOM) And Labrador She may not have been named one of KPMG Professionals Award Karen Branscombe the most powerful women in Canada, but Superintendent, NB School District 02 on December 3, 2010, Kathy Dunderdale Moncton, NB was sworn in as the most powerful politi- cian in Newfoundland and Labrador.