Propagation of Mitochondria-Derived Reactive Oxygen Species Within the Dipodascus Magnusii Cells
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
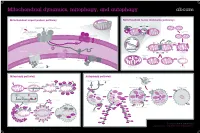
Mitochondrial Dynamics, Mitophagy, and Autophagy
Mitochondrial dynamics, mitophagy, and autophagy Mitochondrial import protein pathway Mitochondrial fusion and fission pathways iΔΨm, (OXPHOSh) Ca2+ Fusion ++++ (Cell differentiationi) N Internal signal β-barrel outer Opa1 SH ER sequence SH membrane precursors Mfn1/2 SH SH GTP GTP C Inner membrane and GTP GTP Outer membrane Matrix carrier precursors 22 70 GTP fusion (N-terminal presequences) 35 GTP 20 37 Tom complex 5 Sam complex Opa1 Cytosol Outer 6 Sam50 7 Mdm10 membrane DRP1 Fis1 Inner membrane fusion Tim8-Tim13 Mim 1 Tom40 Bax/Bak apoptosis Outer membrane HR2 regions S-S Tim9-Tim10 S-S Inner Erv1 95 A 54 mitochondrial S-S Reorganization Mia40 space 21 Fission sequestration 50 18 Tim22 Translocation Depolarization to meet iΔΨm ATP needs Tim23 KREBS Cycle NADH FADH2 17 Oxa1 Tim 22 complex Mia Mba1 Mdm I ATP 44 38 H+ II 17 H+ Inner membrane 16 +++ III ADP - Pi 18 Ribosome Oxa H+ IV mtHsp70 H+ ATP +++ Mge1 MPP Healthy mitochondrion Mitophagy Matrix Tim 23 complex Mitophagy pathway Autophagy pathway Lysosomal hydroiase Lysosome Hypoxia, Nutrient/Growth Rapamycin Starvation condition Ubiquitin Cytosol Parkin factor deprivation Lamp Stress Damaged mitochondrion Parkin Parkin iΔΨm Parkin MARF PINK1 PINK1 PINK1 Mfn1 VDAC1 Parkin AMPK mTOR Mfn2 PINK1 PINK1 Phagophore Autophagosome Autophagolyosome PARL LC3-II PINK1-L PINK1-S Parkin P FIS1 ATG13 ULK LC3-II Membrane PINK1 PINK1 FIP200 PINK1 MIRO2 P Bak PINK1 MIRO1 Parkin Parkin Parkin Cytoplasmic Cytosol proLC3 macromolecule LC3-II Atg4B Atg7/LC3-I Atg5/Atg12/Atg16 Organelle Atg9 PE Atg18 Atg2 -

Alternative Oxidase: a Mitochondrial Respiratory Pathway to Maintain Metabolic and Signaling Homeostasis During Abiotic and Biotic Stress in Plants
Int. J. Mol. Sci. 2013, 14, 6805-6847; doi:10.3390/ijms14046805 OPEN ACCESS International Journal of Molecular Sciences ISSN 1422-0067 www.mdpi.com/journal/ijms Review Alternative Oxidase: A Mitochondrial Respiratory Pathway to Maintain Metabolic and Signaling Homeostasis during Abiotic and Biotic Stress in Plants Greg C. Vanlerberghe Department of Biological Sciences and Department of Cell and Systems Biology, University of Toronto Scarborough, 1265 Military Trail, Toronto, ON, M1C1A4, Canada; E-Mail: [email protected]; Tel.: +1-416-208-2742; Fax: +1-416-287-7676 Received: 16 February 2013; in revised form: 8 March 2013 / Accepted: 12 March 2013 / Published: 26 March 2013 Abstract: Alternative oxidase (AOX) is a non-energy conserving terminal oxidase in the plant mitochondrial electron transport chain. While respiratory carbon oxidation pathways, electron transport, and ATP turnover are tightly coupled processes, AOX provides a means to relax this coupling, thus providing a degree of metabolic homeostasis to carbon and energy metabolism. Beside their role in primary metabolism, plant mitochondria also act as “signaling organelles”, able to influence processes such as nuclear gene expression. AOX activity can control the level of potential mitochondrial signaling molecules such as superoxide, nitric oxide and important redox couples. In this way, AOX also provides a degree of signaling homeostasis to the organelle. Evidence suggests that AOX function in metabolic and signaling homeostasis is particularly important during stress. These include abiotic stresses such as low temperature, drought, and nutrient deficiency, as well as biotic stresses such as bacterial infection. This review provides an introduction to the genetic and biochemical control of AOX respiration, as well as providing generalized examples of how AOX activity can provide metabolic and signaling homeostasis. -

Involvement of Impaired Autophagy and Mitophagy in Neuro-2A Cell
www.nature.com/scientificreports OPEN Involvement of impaired autophagy and mitophagy in Neuro-2a cell damage under hypoxic and/or high- Received: 19 May 2017 Accepted: 15 January 2018 glucose conditions Published: xx xx xxxx Yufei Song, Yu Du, Wenying Zou, Yan Luo, Xiaojie Zhang & Jianliang Fu Chronic cerebral hypoperfusion (CCH) plays an insidious role in the development of cognitive impairment. Considerable evidence suggests that Diabetes Mellitus (DM) as a vascular risk factor may exacerbate CCH and is closely related to cognitive decline. Dysregulation of autophagy is known to be associated with the pathogenesis of neurodegenerative diseases such as Alzheimer’s disease. To elucidate the role of autophagy in CCH- and/or DM-related pathogenesis, mouse neuroblastoma Neuro-2a cells were exposed to hypoxia and/or high glucose for 48 h, mimicking CCH complicated with DM pathologies. Chronic hypoxia reduced cell proliferation and increased levels of cleaved caspase-3, whereas high glucose had no obvious synergistic toxic efect. Accumulation of autophagic vacuoles under hypoxia may be due to both autophagy impairment and induction, with the former accounting for Neuro-2a cell death. Additionally, aberrant accumulation of mitochondria in Neuro-2a cells may be attributed to insufcient BNIP3-mediated mitophagy due to poor interaction between BNIP3 and LC3-II. Despite the lack of a signifcant cytotoxic efect of high glucose under our experimental conditions, our data indicated for the frst time that impaired autophagy degradation and inefcient BNIP3-mediated mitophagy may constitute mechanisms underlying neuronal cell damage during chronic hypoxia. Chronic cerebral hypoperfusion (CCH) is a normal process related to ageing that likely contributes to age-related memory loss1. -

Generation of Reactive Oxygen Species in the Anterior Eye Segment
BBA Clinical 6 (2016) 49–68 Contents lists available at ScienceDirect BBA Clinical journal homepage: www.elsevier.com/locate/bbaclin Review article Generation of reactive oxygen species in the anterior eye segment. Synergistic codrugs of N-acetylcarnosine lubricant eye drops and mitochondria-targeted antioxidant act as a powerful therapeutic platform for the treatment of cataracts and primary open-angle glaucoma☆ Mark A. Babizhayev ⁎ Innovative Vision Products, Inc., 3511 Silverside Road, Suite 105, County of New Castle, DE 19810, USA article info abstract Article history: Senile cataract is a clouding of the lens in the aging eye leading to a decrease in vision. Symptoms may Received 13 January 2016 include faded colors, blurry vision, halos around light, trouble with bright lights, and trouble seeing at Received in revised form 5 April 2016 night. This may result in trouble driving, reading, or recognizing faces. Cataracts are the cause of half of blindness Accepted 11 April 2016 and 33% of visual impairment worldwide. Cataracts result from the deposition of aggregated proteins in the Available online 19 April 2016 eye lens and lens fiber cells plasma membrane damage which causes clouding of the lens, light scattering, Keywords: and obstruction of vision. ROS induced damage in the lens cell may consist of oxidation of proteins, DNA Aging eye damage and/or lipid peroxidation, all of which have been implicated in cataractogenesis. The inner eye pressure Age-related cataracts (also called intraocular pressure or IOP) rises because the correct amount of fluid can't drain out of the eye. Eye lens and aqueous humor With primary open-angle glaucoma, the entrances to the drainage canals are clear and should be working cor- Membrane derangement rectly. -

IDH2 Deficiency Impairs Cutaneous Wound Healing Via ROS-Dependent
BBA - Molecular Basis of Disease 1865 (2019) 165523 Contents lists available at ScienceDirect BBA - Molecular Basis of Disease journal homepage: www.elsevier.com/locate/bbadis IDH2 deficiency impairs cutaneous wound healing via ROS-dependent apoptosis T ⁎ Sung Hwan Kim, Jeen-Woo Park School of Life Sciences and Biotechnology, BK21 Plus KNU Creative BioResearch Group, College of Natural Sciences, Kyungpook National University, Taegu, Republic of Korea ARTICLE INFO ABSTRACT Keywords: Dermal fibroblasts are mesenchymal cells found between the skin epidermis and subcutaneous tissue that play a Wound healing pivotal role in cutaneous wound healing by synthesizing fibronectin (a component of the extracellular matrix), Apoptosis secreting angiogenesis factors, and generating strong contractile forces. In wound healing, low concentrations of IDH2 reactive oxygen species (ROS) are essential in combating invading microorganisms and in cell-survival signaling. Mitochondria However, excessive ROS production impairs fibroblasts. Mitochondrial NADP+-dependent isocitrate dehy- Mito-TEMPO drogenase (IDH2) is a key enzyme that regulates the mitochondrial redox balance and reduces oxidative stress- induced cell injury through the generation of NADPH. In the present study, the downregulation of IDH2 ex- pression resulted in an increase in cell apoptosis in mouse skin through ROS-dependent ATM-mediated p53 signaling. IDH2 deficiency also delayed cutaneous wound healing in mice and impaired dermal fibroblast function. Furthermore, pretreatment with the mitochondria-targeted antioxidant mito-TEMPO alleviated the apoptosis induced by IDH2 deficiency both in vitro and in vivo. Together, our findings highlight the role of IDH2 in cutaneous wound healing in association with mitochondrial ROS. 1. Introduction expression, while also stimulating the proliferation and migration of fibroblasts, leading to the formation of the extracellular matrix (ECM). -

Mitochondrial Rab Gaps Govern Autophagosome Biogenesis During Mitophagy Koji Yamano1, Adam I Fogel1, Chunxin Wang1, Alexander M Van Der Bliek2, Richard J Youle1*
RESEARCH ARTICLE elife.elifesciences.org Mitochondrial Rab GAPs govern autophagosome biogenesis during mitophagy Koji Yamano1, Adam I Fogel1, Chunxin Wang1, Alexander M van der Bliek2, Richard J Youle1* 1Biochemistry Section, Surgical Neurology Branch, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, United States; 2Department of Biological Chemistry, David Geffen School of Medicine at University of California, Los Angeles, Los Angeles, United States Abstract Damaged mitochondria can be selectively eliminated by mitophagy. Although two gene products mutated in Parkinson’s disease, PINK1, and Parkin have been found to play a central role in triggering mitophagy in mammals, how the pre-autophagosomal isolation membrane selectively and accurately engulfs damaged mitochondria remains unclear. In this study, we demonstrate that TBC1D15, a mitochondrial Rab GTPase-activating protein (Rab-GAP), governs autophagosome biogenesis and morphology downstream of Parkin activation. To constrain autophagosome morphogenesis to that of the cargo, TBC1D15 inhibits Rab7 activity and associates with both the mitochondria through binding Fis1 and the isolation membrane through the interactions with LC3/ GABARAP family members. Another TBC family member TBC1D17, also participates in mitophagy and forms homodimers and heterodimers with TBC1D15. These results demonstrate that TBC1D15 and TBC1D17 mediate proper autophagic encapsulation of mitochondria by regulating Rab7 activity at the interface between mitochondria and isolation membranes. DOI: 10.7554/eLife.01612.001 *For correspondence: [email protected] Introduction Competing interests: See page 21 Autophagosomes enclose seemingly random portions of the cytoplasm to supply nutrients during starvation or they can specifically engulf cellular debris to maintain quality control (Mizushima et al., Funding: See page 21 2011). -

Differential Redox-Regulation and Mitochondrial Dynamics in Normal and Leukemic Hematopoietic Stem Cells a Potential Window
Critical Reviews in Oncology / Hematology 144 (2019) 102814 Contents lists available at ScienceDirect Critical Reviews in Oncology / Hematology journal homepage: www.elsevier.com/locate/critrevonc Differential redox-regulation and mitochondrial dynamics in normal and leukemic hematopoietic stem cells: A potential window for leukemia T therapy ⁎ Katharina Mattes, Edo Vellenga, Hein Schepers Department of Hematology, Cancer Research Center Groningen, University Medical Center Groningen, University of Groningen, Groningen, the Netherlands ARTICLE INFO ABSTRACT Keywords: The prognosis for many patients with acute myeloid leukemia (AML) is poor, mainly due to disease relapse HSC driven by leukemia stem cells (LSCs). Recent studies have highlighted the unique metabolic properties of LSCs, LSC which might represent opportunities for LSC-selective targeting. LSCs characteristically have low levels of re- Mitochondria active oxygen species (ROS), which apparently result from a combination of low mitochondrial activity and high ROS activity of ROS-removing pathways such as autophagy. Due to this low activity, LSCs are highly dependent on BCL-2 mitochondrial regulatory mechanisms. These include the anti-apoptotic protein BCL-2, which also has crucial Autophagy Venetoclax roles in regulating the mitochondrial membrane potential, and proteins involved in mitophagy. Here we review the different pathways that impact mitochondrial activity and redox-regulation, and highlight their relevance for the functionality of both HSCs and LSCs. Additionally, novel AML therapy strategies that are based on interference with those pathways, including the promising BCL-2 inhibitor Venetoclax, are summar- ized. 1. Introduction and scope of this review regulating ROS production and mitochondrial health. HSCs rely on glycolysis as their main energy source, which results in less oxidative Current treatment strategies for acute myeloid leukemia (AML) re- burden compared to mitochondrial oxidative phosphorylation (OX- sult in an initial reduction of leukemic blasts in the majority of patients. -

Mitoq Inhibits Hepatic Stellate Cell Activation and Liver Fibrosis by Enhancing PINK1/Parkin-Mediated Mitophagy
MitoQ Inhibits Hepatic Stellate Cell Activation and Liver Fibrosis by Enhancing PINK1/parkin-mediated Mitophagy Shi-Ying Dou Second Hospital of Hebei Medical University Jiu-Na Zhang Second Hospital of Hebei Medical University Xiao-Li Xie Second Hospital of Hebei Medical University Ting Liu Second Hospital of Hebei Medical University Jun-Li Hu Second Hospital of Hebei Medical University Xiao-Yu Jiang Second Hospital of Hebei Medical University Miao-Miao Wang Second Hospital of Hebei Medical University Hui-Qing Jiang ( [email protected] ) Second Hospital of Hebei Medical University https://orcid.org/0000-0002-5235-1457 Research Article Keywords: Liver brosis, hepatic stellate cell, ubiquinone, PINK1 mitophagy Posted Date: March 30th, 2021 DOI: https://doi.org/10.21203/rs.3.rs-344963/v1 License: This work is licensed under a Creative Commons Attribution 4.0 International License. Read Full License Page 1/18 Abstract Mitophagy plays an important role in the activation of hepatic stellate cells (HSCs). Mitochondria- targeted ubiquinone (MitoQ) is a mitochondria-targeted antioxidant that reduces the production of intracellular reactive oxygen species (ROS). However, its relationship with mitophagy remains unclear. This study evaluated mitophagy during HSC activation and the effects of MitoQ on mitophagy in cell culture and in an animal model of the activation of HSCs. We found that MitoQ reduced the activation of HSCs and alleviated hepatic brosis. While activation of primary HSCs or LX-2 cells was associated with reduced PINK1/parkin-mediated mitophagy, MitoQ reduced intracellular ROS levels, enhanced PINK1/parkin-mediated mitophagy, and inhibited the activation of HSCs. After knocking down the key mitophagy-related protein, PINK1, in LX-2 cells to block mitophagy, MitoQ intervention failed to inhibit HSC activation. -

Appoptosin Interacts with Mitochondrial Outer-Membrane Fusion Proteins and Regulates Mitochondrial Morphology
© 2016. Published by The Company of Biologists Ltd | Journal of Cell Science (2016) 129, 994-1002 doi:10.1242/jcs.176792 RESEARCH ARTICLE Appoptosin interacts with mitochondrial outer-membrane fusion proteins and regulates mitochondrial morphology Cuilin Zhang1, Zhun Shi1, Lingzhi Zhang1, Zehua Zhou1, Xiaoyuan Zheng1, Guiying Liu1, Guojun Bu1, Paul E. Fraser2, Huaxi Xu1,3 and Yun-wu Zhang1,* ABSTRACT mitochondrial mobility, mitophagy, cell mitosis and apoptosis Mitochondrial morphology is regulated by fusion and fission (Youle and van der Bliek, 2012). – machinery. Impaired mitochondria dynamics cause various Mitochondrial fusion comprises two events outer-membrane diseases, including Alzheimer’s disease. Appoptosin (encoded by fusion and inner-membrane fusion. In mammalian cells, two SLC25A38) is a mitochondrial carrier protein that is located in the GTPases MFN1 and MFN2, which anchor on the outer mitochondrial inner membrane. Appoptosin overexpression causes membrane of mitochondria, are responsible for the outer- overproduction of reactive oxygen species (ROS) and caspase- membrane fusion (Chen et al., 2003; Koshiba et al., 2004). dependent apoptosis, whereas appoptosin downregulation abolishes However, compared to MFN2, MFN1 is relatively specific in β-amyloid-induced mitochondrial fragmentation and neuronal death regulating mitochondrial fusion (Shen et al., 2007). MFN1- during Alzheimer’s disease. Herein, we found that overexpression harboring mitochondria have a higher tethering efficiency than of appoptosin resulted in mitochondrial fragmentation in a manner those with MFN2, and purified MFN1 also possesses higher independent of its carrier function, ROS production or caspase GTPase activity than MFN2 (Ishihara et al., 2004). In contrast, activation. Although appoptosin did not affect levels of mitochondrial MFN2 is more tissue specific in its expression pattern than MFN1 outer-membrane fusion (MFN1 and MFN2), inner-membrane fusion (Liesa et al., 2009). -

Melatonin Induces Apoptosis in Cholangiocarcinoma Cell Lines by Activating the Reactive Oxygen Species-Mediated Mitochondrial Pathway
ONCOLOGY REPORTS 33: 1443-1449, 2015 Melatonin induces apoptosis in cholangiocarcinoma cell lines by activating the reactive oxygen species-mediated mitochondrial pathway Umawadee LAOTHONG1-3,5, YUSUKE HIRAKU2, SHINJI Oikawa2, KITTI INTUYOD3,4, MARIKO Murata2* and SOMCHAI PINLAOR1,3* 1Department of Parasitology, Faculty of Medicine, Khon Kaen University, Khon Kaen 40002, Thailand; 2Department of Environmental and Molecular Medicine, Mie University Graduate School of Medicine, Mie 514-8507, Japan; 3Liver Fluke and Cholangiocarcinoma Research Center, Faculty of Medicine, Khon Kaen University, Khon Kaen 40002, Thailand; 4Biomedical Science Program, Graduate School, Khon Kaen University, Khon Kaen 40002, Thailand Received November 26, 2014; Accepted January 2, 2015 DOI: 10.3892/or.2015.3738 Abstract. We previously demonstrated that melatonin could pro-oxidant by activating ROS-dependent DNA damage and be used as a chemopreventive agent for inhibiting cholangio- thus leading to the apoptosis of CCA cells. carcinoma (CCA) development in a hamster model. However, the cytotoxic activity of melatonin in cancer remains unclear. Introduction In the present study, we investigated the effect of melatonin on CCA cell lines. Human CCA cell lines (KKU-M055 and Cholangiocarcinoma (CCA) is a devastating biliary cancer that KKU-M214) were treated with melatonin at concentrations poses continuing diagnostic and therapeutic challenges (1). of 0.5, 1 and 2 mM for 48 h. Melatonin treatment exerted a There are several risk factors for CCA: mainly liver fluke cytotoxic effect on CCA cells by inhibiting CCA cell viability infection, primary sclerosing cholangitis, biliary-duct cysts in a concentration-dependent manner. Treatment with mela- and hepatolithiasis (2). The highest prevalence of liver fluke tonin, especially at 2 mM, increased intracellular reactive Opisthorchis viverrini infection has been reported in Northeast oxygen species (ROS) production and in turn led to increased Thailand, where CCA incidence is also high (3). -

Gp78 E3 Ubiquitin Ligase Mediates Both Basal and Damage-Induced Mitophagy
bioRxiv preprint doi: https://doi.org/10.1101/407593; this version posted September 3, 2018. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. Gp78 E3 ubiquitin ligase mediates both basal and damage-induced mitophagy Bharat Joshi, Yahya Mohammadzadeh, Guang Gao and Ivan R. Nabi Department of Cellular and Physiological Sciences, Life Sciences Institute, University of British Columbia, Vancouver, BC V6T 1Z3, Canada #Running title: Gp78 control of mitophagy §To whom correspondence should be addressed: Ivan R. Nabi, Department of Cellular and Physiological Sciences, Life Sciences Institute, University of British Columbia, 2350 Health Sciences Mall, Vancouver, BC V6T 1Z3 Canada. Tel: +1-(604) 822-7000 E-mail: [email protected] Key words: Gp78 ubiquitin ligase; mitochondria; autophagy; PINK1; Parkin bioRxiv preprint doi: https://doi.org/10.1101/407593; this version posted September 3, 2018. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. Abstract Mitophagy, the elimination of mitochondria by the autophagy machinery, evolved to monitor mitochondrial health and maintain mitochondrial integrity. PINK1 is a sensor of mitochondrial health that recruits Parkin and other mitophagy-inducing ubiquitin ligases to depolarized mitochondria. However, mechanisms underlying mitophagic control of mitochondrial homeostasis, basal mitophagy, remain poorly understood. The Gp78 E3 ubiquitin ligase, an endoplasmic reticulum membrane protein, induces mitochondrial fission, endoplasmic reticulum- mitochondria contacts and mitophagy of depolarized mitochondria. CRISPR/Cas9 knockout of Gp78 in HT-1080 fibrosarcoma cells results in reduced ER-mitochondria contacts, increased mitochondrial volume and resistance to CCCP-induced mitophagy. -

Mitochondria's Role in Skin Ageing
biology Review Mitochondria’s Role in Skin Ageing Roisin Stout and Mark Birch-Machin * Dermatological Sciences, Institute of Cellular Medicine, Medical School, Newcastle University, Newcastle upon Tyne NE2 4HH, UK; [email protected] * Correspondence: [email protected] Received: 21 December 2018; Accepted: 7 February 2019; Published: 11 May 2019 Abstract: Skin ageing is the result of a loss of cellular function, which can be further accelerated by external factors. Mitochondria have important roles in skin function, and mitochondrial damage has been found to accumulate with age in skin cells, but also in response to solar light and pollution. There is increasing evidence that mitochondrial dysfunction and oxidative stress are key features in all ageing tissues, including skin. This is directly linked to skin ageing phenotypes: wrinkle formation, hair greying and loss, uneven pigmentation and decreased wound healing. The loss of barrier function during skin ageing increases susceptibility to infection and affects wound healing. Therefore, an understanding of the mechanisms involved is important clinically and also for the development of antiageing skin care products. Keywords: mitochondria; skin; ageing; reactive oxygen species; photoageing 1. Skin Structure Skin is the largest organ of the human body and made up of three distinct layers: the epidermis, the dermis and subcutaneous fat. It functions as a barrier against the environment, providing protection against microbes as well as fluid and temperature homeostasis. The epidermis is a thin layer of densely packed keratinised epithelial cells (keratinocytes) which contains no nerves or blood vessels and relies on the thick dermal layer underneath for metabolism.