(12) Patent Application Publication (10) Pub. No.: US 2008/0103103 A1 Memarzadeh Et Al
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Revised 4/1/2021 GEORGIA MEDICAID FEE-FOR-SERVICE HIV
GEORGIA MEDICAID FEE-FOR-SERVICE HIV-AIDS PA SUMMARY Preferred (may not be all inclusive) Non-Preferred Abacavir generic Abacavir/lamivudine/zidovudine generic Abacavir/lamivudine generic Aptivus (tipranavir) Complera (emtricitabine/rilpivirine/tenofovir disoproxil Atazanavir capsules generic fumarate) Atripla (efavirenz/emtricitabine/tenofovir disoproxil Crixivan (indinavir) fumarate) Biktarvy (bictegravir/emtricitabine/tenofovir Delstrigo (doravirine/lamivudine/tenofovir disoproxil alafenamide) fumarate) Cimduo (lamivudine/tenofovir disoproxil fumarate) Fuzeon (enfuvirtide) Descovy (emtricitabine/tenofovir alafenamide) Intelence (etravirine) Dovato Invirase (saquinavir) Edurant (rilpivirine)* Lexiva (fosamprenavir) Efavirenz tablets generic Nevirapine extended-release generic Emtriva (emtricitabine) Norvir Powder (ritonavir) Epivir solution (lamivudine) Pifeltro (doravirine) Evotaz (atazanavir/cobicistat)* Reyataz Powder (atazanavir) Genvoya (elvitegravir/cobicistat/emtricitabine/ Ritonavir tablets generic tenofovir alafenamide) Isentress and Isentress HD (raltegravir)* Rukobia (fostemsavir) Juluca (dolutegravir/rilpivirine) Selzentry (maraviroc) Kaletra (lopinavir/ritonavir) Stavudine generic^ Stribild (elvitegravir/cobicistat/emtricitabine/ tenofovir Lamivudine generic disoproxil fumarate) Symfi (efavirenz 600 mg/lamivudine/tenofovir Lamivudine/zidovudine generic disoproxil fumarate) Symfi Lo (efavirenz 400 mg/lamivudine/tenofovir Nevirapine immediate-release tablets generic disoproxil fumarate) Norvir (ritonavir) Temixys (lamivudine/tenofovir -

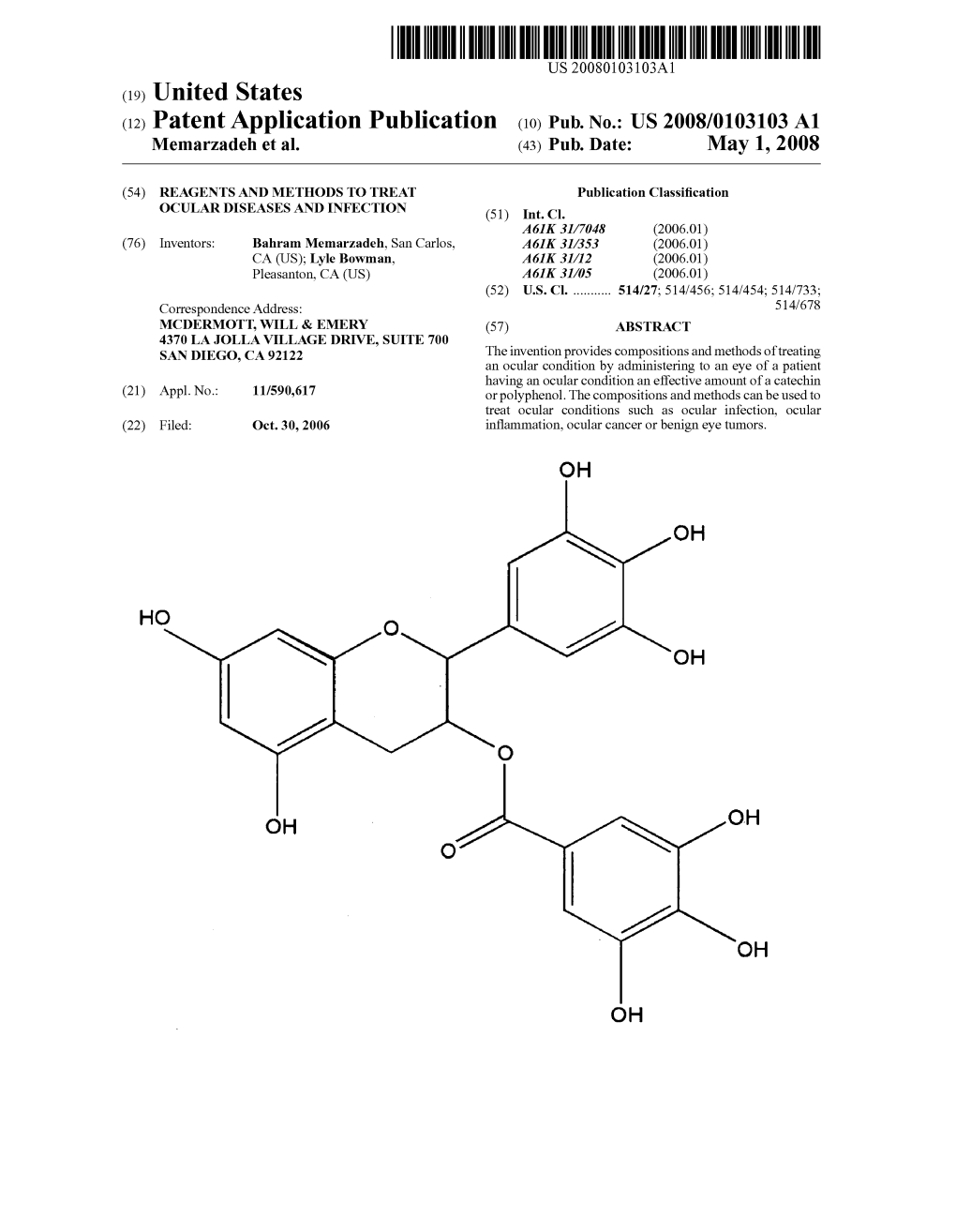
(12) United States Patent (10) Patent No.: US 7,732,399 B2 Goldenberg Et Al
US007732399B2 (12) United States Patent (10) Patent No.: US 7,732,399 B2 Goldenberg et al. (45) Date of Patent: *Jun. 8, 2010 (54) SUSTAINED RELEASE FORMULATIONS 2002fO151582 A1 10, 2002 Dou et al. 2003/O190307 A1* 10, 2003 DiBiase et al. ............. 424,856 (75) Inventors: Merrill S. Goldenberg, Thousand Oaks, 2004/0142048 A1 7/2004 Moore et al. CA (US); Jian Hua Gu, Thousand Oaks 2005/0180925 A1* 8/2005 Chaudry ...................... 424/46 CA (US s s 2005/0215470 A1 9/2005 Ng et al. FOREIGN PATENT DOCUMENTS (73) Assignee: Amgen Inc., Thousand Oaks, CA (US) GB 92.9405 A 6, 1963 (*) Notice: Subject to any disclaimer, the term of this GB 1234.805. A 6, 1971 patent is extended or adjusted under 35 W W 3. t E. U.S.C. 154(b) by 193 days. WO WO99,04764 2, 1999 This patent is Subject to a terminal dis- W W 995, 239, claimer. WO WO 2004/O12522 2, 2004 (21) Appl. No.: 11/847,984 OTHER PUBLICATIONS 1-1. Yan et al. Identification of histatins as tannin-binding proteins in (22) Filed: Aug. 30, 2007 human saliva. Biochemical Journal. 1995, vol. 311, pp. 341-347.* O O Charlton, A. J. et al., “Polyphenol/Peptide Binding and Precipita (65) Prior Publication Data tion.” J. Agric. Food Chem. 50, pp. 1593-1601 (2002); published by US 2007/0292506 A1 Dec. 20, 2007 Chasin,American M.. Chemical “Biodegradable Society. Polymers for Controlled Drug Deliv O O ery,” J.O. Hollinger Editor, Biomedical Applications of Synthetic Related U.S. Application Data Biodegradable Polymers CRC, Boca Raton, FL (1995), pp. -

Minutes of PRAC Meeting on 09-12 July 2018
6 September 2018 EMA/PRAC/576790/2018 Inspections, Human Medicines Pharmacovigilance and Committees Division Pharmacovigilance Risk Assessment Committee (PRAC) Minutes of the meeting on 09-12 July 2018 Chair: June Raine – Vice-Chair: Almath Spooner Health and safety information In accordance with the Agency’s health and safety policy, delegates were briefed on health, safety and emergency information and procedures prior to the start of the meeting. Disclaimers Some of the information contained in the minutes is considered commercially confidential or sensitive and therefore not disclosed. With regard to intended therapeutic indications or procedure scope listed against products, it must be noted that these may not reflect the full wording proposed by applicants and may also change during the course of the review. Additional details on some of these procedures will be published in the PRAC meeting highlights once the procedures are finalised. Of note, the minutes are a working document primarily designed for PRAC members and the work the Committee undertakes. Note on access to documents Some documents mentioned in the minutes cannot be released at present following a request for access to documents within the framework of Regulation (EC) No 1049/2001 as they are subject to on- going procedures for which a final decision has not yet been adopted. They will become public when adopted or considered public according to the principles stated in the Agency policy on access to documents (EMA/127362/2006, Rev. 1). 30 Churchill Place ● Canary Wharf ● London E14 5EU ● United Kingdom Telephone +44 (0)20 3660 6000 Facsimile +44 (0)20 3660 5555 Send a question via our website www.ema.europa.eu/contact An agency of the European Union © European Medicines Agency, 2018. -

EVOTAZ (Atazanavir Or Cobicistat) to Pregnant Rats and Rabbits (See Data)
HIGHLIGHTS OF PRESCRIBING INFORMATION • Assess creatinine clearance (CLcr) before initiating treatment. Consider These highlights do not include all the information needed to use EVOTAZ alternative medications that do not require dosage adjustments in patients safely and effectively. See full prescribing information for EVOTAZ. with renal impairment. (5.3) • When cobicistat, a component of EVOTAZ, is used in combination with a EVOTAZ (atazanavir and cobicistat) tablet, for oral use tenofovir disoproxil fumarate (tenofovir DF)-containing regimen, cases of Initial U.S. Approval: 2015 acute renal failure and Fanconi syndrome have been reported. (5.4) • When used with tenofovir DF, assess urine glucose and urine protein at ---------------------------RECENT MAJOR CHANGES-------------------------- baseline and monitor CLcr, urine glucose, and urine protein. Monitor serum Indications and Usage phosphorus in patients with or at risk for renal impairment. Coadministration Indications (1.1) 07/2020 with tenofovir DF is not recommended in patients with CLcr below 70 Dosage and Administration mL/min or in patients also receiving a nephrotoxic agent. (5.4) Laboratory Testing Prior to Initiation and During • Chronic kidney disease has been reported during postmarketing surveillance Treatment with EVOTAZ (2.1) 04/2020 in patients with HIV-1 infection treated with atazanavir, with or without Recommended Dosage (2.2) 07/2020 ritonavir. Consider alternatives in patients at high risk for renal disease or Not Recommended During Pregnancy (2.5) 04/2020 with preexisting renal disease. Monitor renal laboratory tests prior to therapy Contraindications (4) 04/2020 and during treatment with EVOTAZ. Consider discontinuation of EVOTAZ Warnings and Precautions in patients with progressive renal disease. (5.5) Immune Reconstitution Syndrome (5.11) 07/2020 • Nephrolithiasis and cholelithiasis have been reported. -

Injectable Antiretroviral Drugs: Back to the Future
viruses Review Injectable Antiretroviral Drugs: Back to the Future Marco Berruti 1 , Niccolò Riccardi 2, Diana Canetti 3,4 , Sergio Lo Caputo 5 , Lucia Taramasso 6 and Antonio Di Biagio 1,6,* 1 Infectious Diseases Unit, Department of Health Sciences (DISSAL), University of Genoa, 16132 Genoa, Italy; [email protected] 2 Department of Infectious-Tropical Diseases and Microbiology, IRCCS Sacro Cuore Don Calabria Hospital, Negrar di Valpolicella, 37024 Verona, Italy; [email protected] 3 Clinic of Infectious Diseases, IRCCS San Raffaele Scientific Institute, 20097 Milan, Italy; [email protected] 4 School of Medicine, Vita-Salute San Raffaele University, 20097 Milan, Italy 5 Clinic of Infectious Diseases, Department of Clinical and Experimental Medicine, University of Foggia, 71122 Foggia, Italy; [email protected] 6 Infectious Diseases Unit, Department of Internal Medicine, Ospedale Policlinico San Martino IRCCS, 16132 Genoa, Italy; [email protected] * Correspondence: [email protected] Abstract: Current HIV treatment regimens provide sustained virologic suppression, at least partially restore the immune system and have limited side effects; however, they do not allow viral eradication and they are burdened by daily pill intake with a life-long commitment for the people living with HIV (PHIV). Injectable agents might represent a turning point in the care of PHIV, allowing less frequent administration of antiretroviral treatment (ART), more widespread use of pre-exposure prophylaxis (PrEP) and more stable drug levels in the blood, thus increasing the odds to get closer to end the HIV pandemic. The aim of this manuscript is to give a comprehensive review of injectable antiretrovirals that have been used in the past, which are available now, will be available in the future, and their role in the treatment of HIV infection Citation: Berruti, M.; Riccardi, N.; Canetti, D.; Lo Caputo, S.; Taramasso, Keywords: ART; injectable; HIV; antiretroviral; treatment; PrEP L.; Di Biagio, A. -

Guidelines for the Use of Antiretroviral Agents in Adults and Adolescent Living With
Guidelines for the Use of Antiretroviral Agents in Adults and Adolescents with HIV Developed by the DHHS Panel on Antiretroviral Guidelines for Adults and Adolescents – A Working Group of the Office of AIDS Research Advisory Council (OARAC) How to Cite the Adult and Adolescent Guidelines: Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the Use of Antiretroviral Agents in Adults and Adolescents with HIV. Department of Health and Human Services. Available at https://clinicalinfo.hiv.gov/sites/default/files/guidelines/documents/ AdultandAdolescentGL.pdf. Accessed [insert date] [insert page number, table number, etc. if applicable] It is emphasized that concepts relevant to HIV management evolve rapidly. The Panel has a mechanism to update recommendations on a regular basis, and the most recent information is available on the HIVinfo Web site (http://hivinfo.nih.gov). What’s New in the Guidelines? August 16, 2021 Hepatitis C Virus/HIV Coinfection • Table 18 of this section has been updated to include recommendations regarding concomitant use of fostemsavir or long acting cabotegravir plus rilpivirine with different hepatitis C treatment regimens. June 3, 2021 What to Start • Since the release of the last guidelines, updated data from the Botswana Tsepamo study have shown that the prevalence of neural tube defects (NTD) associated with dolutegravir (DTG) use during conception is much lower than previously reported. Based on these new data, the Panel now recommends that a DTG-based regimen can be prescribed for most people with HIV who are of childbearing potential. Before initiating a DTG-based regimen, clinicians should discuss the risks and benefits of using DTG with persons of childbearing potential, to allow them to make an informed decision. -

Complera Pi.Pdf
HIGHLIGHTS OF PRESCRIBING INFORMATION Patients should currently be on their first or second antiretroviral These highlights do not include all the information needed to use regimen prior to switching therapy. COMPLERA safely and effectively. See full prescribing Patients should have no current or past history of resistance to information for COMPLERA. any of the three components of COMPLERA. COMPLERA® (emtricitabine, rilpivirine, tenofovir disoproxil Additional monitoring of HIV-1 RNA and regimen tolerability is fumarate) tablets, for oral use recommended after replacing therapy to assess for potential virologic Initial U.S. Approval: 2011 failure or rebound. (1) -----------------------DOSAGE AND ADMINISTRATION----------------------- WARNING: LACTIC ACIDOSIS/SEVERE HEPATOMEGALY WITH STEATOSIS and POST TREATMENT ACUTE EXACERBATION Dose in patients 12 years of age and older: One tablet (containing OF HEPATITIS B 200 mg of emtricitabine, 25 mg of rilpivirine, and 300 mg of See full prescribing information for complete boxed warning. tenofovir disoproxil fumarate) taken once daily with food. (2) Dose in renal impairment: Should not be administered in patients Lactic acidosis and severe hepatomegaly with steatosis, with estimated creatinine clearance below 50 mL per minute. (2) including fatal cases, have been reported with the use of nucleoside analogs, including tenofovir disoproxil With rifabutin coadministration, an additional 25 mg tablet of fumarate, a component of COMPLERA. (5.1) rilpivirine (Edurant) once per day is recommended -

Interactions with Protease Inhibitors Charts Revised February 2018
www.hiv-druginteractions.org Interactions with Protease Inhibitors Charts revised February 2018. Full information available at www.hiv-druginteractions.org Page 1 of 4 Please note that if a drug is not listed it cannot automatically be assumed it is safe to coadminister. ATV Cobi DRV FPV IDV LPV RTV SQV TPV ATV Cobi DRV FPV IDV LPV RTV SQV TPV Anaesthetics & Muscle Relaxants Antibacterials (continued) Alcuronium Ertapenem Bupivacaine Erythromycin Cisatracurium Ethambutol Desflurane Ethionamide Dexmedetomidine Flucloxacillin Enflurane Gentamicin Ephedrine Imipenem/Cilastatin Halothane Isoniazid Isoflurane Kanamycin Ketamine Levofloxacin Linezolid Nitrous oxide Meropenem Propofol Metronidazole Rocuronium Moxifloxacin Sevoflurane Nitrofurantoin Sufentanil Ofloxacin Suxamethonium (succinylcholine) Para-aminosalicylic acid Tetracaine Penicillins Thiopental for distribution. for Pyrazinamide Tizanidine Rifabutin Vecuronium Rifampicin Analgesics Rifapentine Alfentanil Rifaximin Aspirin Spectinomycin Buprenorphine Streptomycin Celecoxib Sulfadiazine Codeine Telithromycin Tetracyclines -

Enfuvirtide (Fuzeon) Reference Number: ERX.SPA.196 Effective Date: 01.11.17 Last Review Date: 08.20 Line of Business: Commercial, Medicaid Revision Log
Clinical Policy: Enfuvirtide (Fuzeon) Reference Number: ERX.SPA.196 Effective Date: 01.11.17 Last Review Date: 08.20 Line of Business: Commercial, Medicaid Revision Log See Important Reminder at the end of this policy for important regulatory and legal information. Description Enfuvirtide (Fuzeon®) is a human immunodeficiency virus-1 (HIV-1) fusion inhibitor. FDA Approved Indication(s) Fuzeon is indicated for use in combination with other antiretroviral agents for the treatment of HIV-1 infection in treatment-experienced patients with HIV-1 replication despite ongoing antiretroviral therapy. Policy/Criteria Provider must submit documentation (such as office chart notes, lab results or other clinical information) supporting that member has met all approval criteria. Health plan approved formularies should be reviewed for all coverage determinations. Requirements to use preferred alternative agents apply only when such requirements align with the health plan approved formulary. It is the policy of health plans affiliated with Envolve Pharmacy Solutions™ that Fuzeon is medically necessary when the following criteria are met: I. Initial Approval Criteria A. HIV-1 Infection (must meet all): 1. Diagnosis of HIV-1 infection; 2. Prescribed by or in consultation with an infectious disease or HIV specialist; 3. Age ≥ 6 years; 4. Failure of ≥ 12 weeks of antiretroviral therapy which includes 2 nucleoside analogue reverse transcriptase inhibitors and 1 drug from one of the following classes: an integrase strand transfer inhibitor, a nonnucleoside analogue reverse transcriptase inhibitor, or a pharmacokinetic enhanced protease inhibitor; 5. Current (within the past 30 days) HIV ribonucleic acid viral load ≥ 200 copies/mL; 6. Fuzeon is prescribed concurrently with additional antiretroviral agents to which the member is susceptible; 7. -

Product Information
DATA SHEET 1. EVIPLERA® (TENOFOVIR DISOPROXIL FUMARATE, EMTRICITABINE AND RILPIVIRINE) TABLETS 2. QUALITATIVE AND QUANTITATIVE COMPOSITION 300 mg tenofovir disoproxil fumarate/200 mg emtricitabine/25 mg rilpivirine For full list of excipients, see section 6.1. 3. PHARMACEUTICAL FORM Film-coated tablet. EVIPLERA tablets are capsule shaped, film-coated and purplish-pink in colour. Each tablet is debossed with ‘GSI’ on one side and plain on the other side. 4. CLINICAL PARTICULARS 4.1 Therapeutic Indications EVIPLERA is indicated for the treatment of HIV infection in treatment-naïve adult patients with plasma HIV-1 RNA ≤ 100,000 copies/mL at the start of therapy. EVIPLERA is also indicated in certain virologically-suppressed (HIV-1 RNA <50 copies/mL) adult patients on a stable antiretroviral regimen at start of therapy in order to replace their current antiretroviral treatment regimen (see section 5.1 Pharmacodynamic properties, Clinical trials). Patients must not have a history of resistance to any of the components of EVIPLERA (tenofovir DF, emtricitabine or rilpivirine). 4.2 Dose and method of administration Adults: The recommended dose of EVIPLERA is one tablet once daily taken orally with food. When discontinuation of EVIPLERA is necessary due to one of the components, or where dose modification is necessary, separate preparations of tenofovir DF, emtricitabine and rilpivirine should be used. Please refer to the product information for these products (see section 4.5 Interactions with other medicines and other forms of interactions). Paediatric population: EVIPLERA is not recommended for use in children below 18 years of age due to insufficient data on safety and efficacy. -

FUZEON™™ (Enfuvirtide)
FUZEONä (enfuvirtide) for Injection DESCRIPTION FUZEON (enfuvirtide) is an inhibitor of the fusion of HIV-1 with CD4+ cells. Enfuvirtide is a linear 36-amino acid synthetic peptide with the N-terminus acetylated and the C-terminus is a carboxamide. It is composed of naturally occurring L-amino acid residues. Enfuvirtide is a white to off-white amorphous solid. It has negligible solubility in pure water and the solubility increases in aqueous buffers (pH 7.5) to 85-142 g/100 mL. The empirical formula of enfuvirtide is C204H301N51O64, and the molecular weight is 4492. It has the following primary amino acid sequence: CH3CO-Tyr-Thr-Ser-Leu-Ile-His-Ser-Leu-Ile-Glu-Glu-Ser-Gln-Asn-Gln-Gln-Glu-Lys- Asn-Glu-Gln-Glu-Leu-Leu-Glu-Leu-Asp-Lys-Trp-Ala-Ser-Leu-Trp-Asn-Trp-Phe-NH2 and the following structural formula: O NH2 O NH2 O OH HO HO HO O O O O O O O O H H H H H H H N N N N N N N N N N N N N N N H H H H H H H H O NH O O O O O O O H N H N 2 2 HO HO O N O NH O HN O OH OH O NH O O H N 2 HN O O H N NH 2 HO NH O HO 2 O O HN O N O O O O O H NH H H H H H H N N N N N N N N N N N H N O H H H H H O 2 O O O O O O OH HN O OH O NH NH O 2 HO O HN O N H N H O NH2 The drug product, FUZEON (enfuvirtide) for Injection, is a white to off-white, sterile, lyophilized powder. -

Managing Side Effects of HIV Medications | 1 Managing Side Effects of HIV Medications | 2 Your Doctor Can Also Help You Prepare for Any Side Effects
Managing Side Eects of HIV MEDICATIONS Table of Contents What are side effects?..........................................................................................1 Side effects of different classes of HIV drugs ....................................................9 Why do I need to know about side effects of HIV medications? ......................2 Protease inhibitors ..................................................................................................................9 Fat problems (lipodystrophy) ..............................................................................................9 What questions should I ask when my doctor first prescribes an HIV medication? .............................................................................................2 Metabolic problems ................................................................................................................9 Make sure you understand what the medication does and the best way Heart disease, heart attacks, stroke ...................................................................................9 to take it ......................................................................................................................................2 Diabetes ................................................................................................................................... 10 Your doctor can also help you prepare for any side effects .....................................3 Nucleoside Reverse Transcriptase Inhibitors (NRTIs) .............................................