Exclusionary Methods to Reduce Predation on Ground Nesting Birds
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Piping Plover Fact Sheet
WILDLIFE IN CONNECTICUT STATE THREATENED SPECIES PIPING PLOVER Charadrius melodus Background Carolina to Texas and into eastern Mexico and the In the late 1800s, unrestricted market hunting for Caribbean. the millinery (hat) trade devastated the piping plover Description population on the Atlantic Coast. Not only were the feathers used to adorn women’s hats, but the birds were Piping plovers are white below and creamy brown above, also used for human consumption. Following passage of the color of dry sand. During the breeding season, the Migratory Bird Treaty Act in 1918, the piping plover they have a single black neck band that is sometimes population recovered to a 20th century peak in the incomplete and a black bar above the white forehead. 1940s, only to decline again as human development and This black neck band is completely lacking in winter. recreational use greatly intensified in coastal habitats. The primary feathers are dark brown, while the rump is The population decline led to federal Endangered white, contrasting with the brown back and tail, which Species Act protection in 1986 and Connecticut are very conspicuous in the bird’s distraction display. The Endangered Species Act Protection in 1992. bill is orange with a black tip; the feet are also orange. Adult piping plovers typically weigh between 1.5 to 2.25 The U.S. Fish and Wildlife Service recognizes 3 ounces, and measure in length from 6 to 7 inches with a populations of piping plovers – the Atlantic Coast (which wingspan of 14 to 15.25 inches. includes Connecticut), Great Lakes, and Northern Great Plains. -

New York and New York and Long Island Field Offices Strategic Plan
New York and Long Island Field Offices Strategic Plan FY2012 Table of Contents Page Strategic Plan Introduction 6 New York Focal Area Map 8 ALLEGHENY FOCAL AREA 9 Allegheny Focal Area Map 10 Bald Eagle Species Action Plan 11 Broad-winged Hawk Species Action Plan 19 Brook Trout Species Action Plan 27 Cerulean Warbler Species Action Plan 35 Clubshell Species Action Plan 43 Eastern Hellbender Species Action Plan 51 Rayed Bean Species Action Plan 63 Spotted Darter Species Action Plan 70 FINGER LAKES ONONDAGA FOCAL AREA 78 Finger Lakes/Onondaga Focal Area Map 79 American Hart’s-tongue Fern Species Action Plan 80 American Black Duck Species Action Plan 86 Bog Turtle Species Action Plan 95 Brook Trout Species Action Plan 103 Cerulean Warbler Species Action Plan 113 Chittenango Ovate Amber Snail Species Action Plan 122 Indiana Bat Species Action Plan 129 Lake Sturgeon Species Action Plan 139 Leedy’s Roseroot Species Action Plan 148 Massasauga Rattlesnake Species Action Plan 154 GREAT LAKES FOCAL AREA 160 Great Lakes Focal Area Map 162 American Woodcock Species Action Plan 163 ii Bald Eagle Species Action Plan 173 American Black Duck Species Action Plan 182 Bobolink Species Action Plan 192 Bog Turtle Species Action Plan 199 Broad-winged Hawk Species Action Plan 205 Brook Trout Species Action Plan 212 Cerulean Warbler Species Action Plan 221 Common Tern Species Action Plan 229 Houghton’s Goldenrod Species Action Plan 237 Indiana Bat Species Action Plan 244 Lake Sturgeon Species Action Plan 253 Massasauga Rattlesnake Species Action Plan 262 Piping -

The Birds of New York State
__ Common Goldeneye RAILS, GALLINULES, __ Baird's Sandpiper __ Black-tailed Gull __ Black-capped Petrel Birds of __ Barrow's Goldeneye AND COOTS __ Little Stint __ Common Gull __ Fea's Petrel __ Smew __ Least Sandpiper __ Short-billed Gull __ Cory's Shearwater New York State __ Clapper Rail __ Hooded Merganser __ White-rumped __ Ring-billed Gull __ Sooty Shearwater __ King Rail © New York State __ Common Merganser __ Virginia Rail Sandpiper __ Western Gull __ Great Shearwater Ornithological __ Red-breasted __ Corn Crake __ Buff-breasted Sandpiper __ California Gull __ Manx Shearwater Association Merganser __ Sora __ Pectoral Sandpiper __ Herring Gull __ Audubon's Shearwater Ruddy Duck __ Semipalmated __ __ Iceland Gull __ Common Gallinule STORKS Sandpiper www.nybirds.org GALLINACEOUS BIRDS __ American Coot __ Lesser Black-backed __ Wood Stork __ Northern Bobwhite __ Purple Gallinule __ Western Sandpiper Gull FRIGATEBIRDS DUCKS, GEESE, SWANS __ Wild Turkey __ Azure Gallinule __ Short-billed Dowitcher __ Slaty-backed Gull __ Magnificent Frigatebird __ Long-billed Dowitcher __ Glaucous Gull __ Black-bellied Whistling- __ Ruffed Grouse __ Yellow Rail BOOBIES AND GANNETS __ American Woodcock Duck __ Spruce Grouse __ Black Rail __ Great Black-backed Gull __ Brown Booby __ Wilson's Snipe __ Fulvous Whistling-Duck __ Willow Ptarmigan CRANES __ Sooty Tern __ Northern Gannet __ Greater Prairie-Chicken __ Spotted Sandpiper __ Bridled Tern __ Snow Goose __ Sandhill Crane ANHINGAS __ Solitary Sandpiper __ Least Tern __ Ross’s Goose __ Gray Partridge -

OWLS of OHIO C D G U I D E B O O K DIVISION of WILDLIFE Introduction O W L S O F O H I O
OWLS OF OHIO c d g u i d e b o o k DIVISION OF WILDLIFE Introduction O W L S O F O H I O Owls have longowls evoked curiosity in In the winter of of 2002, a snowy ohio owl and stygian owl are known from one people, due to their secretive and often frequented an area near Wilmington and two Texas records, respectively. nocturnal habits, fierce predatory in Clinton County, and became quite Another, the Oriental scops-owl, is behavior, and interesting appearance. a celebrity. She was visited by scores of known from two Alaska records). On Many people might be surprised by people – many whom had never seen a global scale, there are 27 genera of how common owls are; it just takes a one of these Arctic visitors – and was owls in two families, comprising a total bit of knowledge and searching to find featured in many newspapers and TV of 215 species. them. The effort is worthwhile, as news shows. A massive invasion of In Ohio and abroad, there is great owls are among our most fascinating northern owls – boreal, great gray, and variation among owls. The largest birds, both to watch and to hear. Owls Northern hawk owl – into Minnesota species in the world is the great gray are also among our most charismatic during the winter of 2004-05 became owl of North America. It is nearly three birds, and reading about species with a major source of ecotourism for the feet long with a wingspan of almost 4 names like fearful owl, barking owl, North Star State. -

SHOREBIRDS (Charadriiformes*) CARE MANUAL *Does Not Include Alcidae
SHOREBIRDS (Charadriiformes*) CARE MANUAL *Does not include Alcidae CREATED BY AZA CHARADRIIFORMES TAXON ADVISORY GROUP IN ASSOCIATION WITH AZA ANIMAL WELFARE COMMITTEE Shorebirds (Charadriiformes) Care Manual Shorebirds (Charadriiformes) Care Manual Published by the Association of Zoos and Aquariums in association with the AZA Animal Welfare Committee Formal Citation: AZA Charadriiformes Taxon Advisory Group. (2014). Shorebirds (Charadriiformes) Care Manual. Silver Spring, MD: Association of Zoos and Aquariums. Original Completion Date: October 2013 Authors and Significant Contributors: Aimee Greenebaum: AZA Charadriiformes TAG Vice Chair, Monterey Bay Aquarium, USA Alex Waier: Milwaukee County Zoo, USA Carol Hendrickson: Birmingham Zoo, USA Cindy Pinger: AZA Charadriiformes TAG Chair, Birmingham Zoo, USA CJ McCarty: Oregon Coast Aquarium, USA Heidi Cline: Alaska SeaLife Center, USA Jamie Ries: Central Park Zoo, USA Joe Barkowski: Sedgwick County Zoo, USA Kim Wanders: Monterey Bay Aquarium, USA Mary Carlson: Charadriiformes Program Advisor, Seattle Aquarium, USA Sara Perry: Seattle Aquarium, USA Sara Crook-Martin: Buttonwood Park Zoo, USA Shana R. Lavin, Ph.D.,Wildlife Nutrition Fellow University of Florida, Dept. of Animal Sciences , Walt Disney World Animal Programs Dr. Stephanie McCain: AZA Charadriiformes TAG Veterinarian Advisor, DVM, Birmingham Zoo, USA Phil King: Assiniboine Park Zoo, Canada Reviewers: Dr. Mike Murray (Monterey Bay Aquarium, USA) John C. Anderson (Seattle Aquarium volunteer) Kristina Neuman (Point Blue Conservation Science) Sarah Saunders (Conservation Biology Graduate Program,University of Minnesota) AZA Staff Editors: Maya Seaman, MS, Animal Care Manual Editing Consultant Candice Dorsey, PhD, Director of Animal Programs Debborah Luke, PhD, Vice President, Conservation & Science Cover Photo Credits: Jeff Pribble Disclaimer: This manual presents a compilation of knowledge provided by recognized animal experts based on the current science, practice, and technology of animal management. -

Coastal Courtship and Roadside Romance
Every spring, flocks of shorebirds migrate north to breed. Every spring, some of these migrants settle down and nest here in North Carolina. These small shorebirds come for some VISUAL HUNTERS All plovers are visual hunters, chasing prey on the ground. Piping plovers forage for invertebrates such as marine worms, insects, small crustaceans, mollusks and Coastal Courtship and marine animals’ eggs. A plover stands tall to scan the beach. Then, with short dashes and pecks along the water’s edge, it pursues its prey. After a wave recedes, leaving a film of water on the beach, a plover often holds one Roadside Romance foot out and vibrates it against the wet surface before pecking. This may stir a hidden invertebrate, and the plover sees its written and illustrated by Anne M. Runyon movement under the surface. Wilson’s plovers hunt for fiddler crabs during low tide on mud or sand flats. A hungry hunter grabs one leg of a crab with its stout bill, and shakes till the crab loses its leg and falls. Then the plover grabs lovers are small- to medium-sized shorebirds in the large Charadri- another leg and shakes again. Soon the fiddler crab has no legs left and … Pidae family. They have rounded heads, large eyes, short stout bills and gulp!, the plover eats the crab. medium-length legs. Seven of North America’s 12 plover species visit The plaintive call “killdeer! killdeer! killdeer!” makes killdeer the noisiest killdeer North Carolina during their fall or spring migrations. Some fly from South of our three nesting plovers. -

Alpha Codes for 2168 Bird Species (And 113 Non-Species Taxa) in Accordance with the 62Nd AOU Supplement (2021), Sorted Taxonomically
Four-letter (English Name) and Six-letter (Scientific Name) Alpha Codes for 2168 Bird Species (and 113 Non-Species Taxa) in accordance with the 62nd AOU Supplement (2021), sorted taxonomically Prepared by Peter Pyle and David F. DeSante The Institute for Bird Populations www.birdpop.org ENGLISH NAME 4-LETTER CODE SCIENTIFIC NAME 6-LETTER CODE Highland Tinamou HITI Nothocercus bonapartei NOTBON Great Tinamou GRTI Tinamus major TINMAJ Little Tinamou LITI Crypturellus soui CRYSOU Thicket Tinamou THTI Crypturellus cinnamomeus CRYCIN Slaty-breasted Tinamou SBTI Crypturellus boucardi CRYBOU Choco Tinamou CHTI Crypturellus kerriae CRYKER White-faced Whistling-Duck WFWD Dendrocygna viduata DENVID Black-bellied Whistling-Duck BBWD Dendrocygna autumnalis DENAUT West Indian Whistling-Duck WIWD Dendrocygna arborea DENARB Fulvous Whistling-Duck FUWD Dendrocygna bicolor DENBIC Emperor Goose EMGO Anser canagicus ANSCAN Snow Goose SNGO Anser caerulescens ANSCAE + Lesser Snow Goose White-morph LSGW Anser caerulescens caerulescens ANSCCA + Lesser Snow Goose Intermediate-morph LSGI Anser caerulescens caerulescens ANSCCA + Lesser Snow Goose Blue-morph LSGB Anser caerulescens caerulescens ANSCCA + Greater Snow Goose White-morph GSGW Anser caerulescens atlantica ANSCAT + Greater Snow Goose Intermediate-morph GSGI Anser caerulescens atlantica ANSCAT + Greater Snow Goose Blue-morph GSGB Anser caerulescens atlantica ANSCAT + Snow X Ross's Goose Hybrid SRGH Anser caerulescens x rossii ANSCAR + Snow/Ross's Goose SRGO Anser caerulescens/rossii ANSCRO Ross's Goose -

Checklist of Amphibians, Reptiles, Birds and Mammals of New York
CHECKLIST OF AMPHIBIANS, REPTILES, BIRDS AND MAMMALS OF NEW YORK STATE Including Their Legal Status Eastern Milk Snake Moose Blue-spotted Salamander Common Loon New York State Artwork by Jean Gawalt Department of Environmental Conservation Division of Fish and Wildlife Page 1 of 30 February 2019 New York State Department of Environmental Conservation Division of Fish and Wildlife Wildlife Diversity Group 625 Broadway Albany, New York 12233-4754 This web version is based upon an original hard copy version of Checklist of the Amphibians, Reptiles, Birds and Mammals of New York, Including Their Protective Status which was first published in 1985 and revised and reprinted in 1987. This version has had substantial revision in content and form. First printing - 1985 Second printing (rev.) - 1987 Third revision - 2001 Fourth revision - 2003 Fifth revision - 2005 Sixth revision - December 2005 Seventh revision - November 2006 Eighth revision - September 2007 Ninth revision - April 2010 Tenth revision – February 2019 Page 2 of 30 Introduction The following list of amphibians (34 species), reptiles (38), birds (474) and mammals (93) indicates those vertebrate species believed to be part of the fauna of New York and the present legal status of these species in New York State. Common and scientific nomenclature is as according to: Crother (2008) for amphibians and reptiles; the American Ornithologists' Union (1983 and 2009) for birds; and Wilson and Reeder (2005) for mammals. Expected occurrence in New York State is based on: Conant and Collins (1991) for amphibians and reptiles; Levine (1998) and the New York State Ornithological Association (2009) for birds; and New York State Museum records for terrestrial mammals. -

54971 GPNC Shorebirds
A P ocket Guide to Great Plains Shorebirds Third Edition I I I By Suzanne Fellows & Bob Gress Funded by Westar Energy Green Team, The Nature Conservancy, and the Chickadee Checkoff Published by the Friends of the Great Plains Nature Center Table of Contents • Introduction • 2 • Identification Tips • 4 Plovers I Black-bellied Plover • 6 I American Golden-Plover • 8 I Snowy Plover • 10 I Semipalmated Plover • 12 I Piping Plover • 14 ©Bob Gress I Killdeer • 16 I Mountain Plover • 18 Stilts & Avocets I Black-necked Stilt • 19 I American Avocet • 20 Hudsonian Godwit Sandpipers I Spotted Sandpiper • 22 ©Bob Gress I Solitary Sandpiper • 24 I Greater Yellowlegs • 26 I Willet • 28 I Lesser Yellowlegs • 30 I Upland Sandpiper • 32 Black-necked Stilt I Whimbrel • 33 Cover Photo: I Long-billed Curlew • 34 Wilson‘s Phalarope I Hudsonian Godwit • 36 ©Bob Gress I Marbled Godwit • 38 I Ruddy Turnstone • 40 I Red Knot • 42 I Sanderling • 44 I Semipalmated Sandpiper • 46 I Western Sandpiper • 47 I Least Sandpiper • 48 I White-rumped Sandpiper • 49 I Baird’s Sandpiper • 50 ©Bob Gress I Pectoral Sandpiper • 51 I Dunlin • 52 I Stilt Sandpiper • 54 I Buff-breasted Sandpiper • 56 I Short-billed Dowitcher • 57 Western Sandpiper I Long-billed Dowitcher • 58 I Wilson’s Snipe • 60 I American Woodcock • 61 I Wilson’s Phalarope • 62 I Red-necked Phalarope • 64 I Red Phalarope • 65 • Rare Great Plains Shorebirds • 66 • Acknowledgements • 67 • Pocket Guides • 68 Supercilium Median crown Stripe eye Ring Nape Lores upper Mandible Postocular Stripe ear coverts Hind Neck Lower Mandible ©Dan Kilby 1 Introduction Shorebirds, such as plovers and sandpipers, are a captivating group of birds primarily adapted to live in open areas such as shorelines, wetlands and grasslands. -

SC Priority Species SC CWCS
Chapter 2: SC Priority Species SC CWCS CHAPTER 2: SOUTH CAROLINA PRIORITY SPECIES The State Wildlife Grants program established funding for species not traditionally covered under federal funding programs. To qualify for these funds, each state was mandated to develop a Strategy with a focus on “species of greatest conservation concern;” guidance was provided to the states to begin identifying these species. SCDNR recognized the importance of including species that are currently rare or designated as at-risk, those for which we have knowledge deficiencies and those that have not received adequate conservation attention in the past. Additionally, SCDNR included species for which South Carolina is “responsible,” that is, species that may be common in our state, but are declining or rare elsewhere. SCDNR also included species that could be used as indicators of detrimental conditions. These indicator species may be common in South Carolina; as such, changes in their population status are likely to indicate stress to other species that occur in the same habitat. The diversity of animals in South Carolina is vast. Habitats in this state range from the mountains to the ocean and include many different taxonomic animal groups. SCDNR wanted to address as many of those groups as possible for inclusion in the list of priority species for the CWCS; as such, twelve taxonomic groups are included in the Strategy: mammals, birds, reptiles, amphibians, freshwater fishes, diadromous fishes, marine fishes, marine invertebrates, crayfish, freshwater mussels, freshwater snails, and insects (both freshwater and terrestrial). However, taxonomic groups that are excluded from this version of the SC CWCS may be included in future revisions of the Strategy, as additional information and experts specific to those groups are identified. -
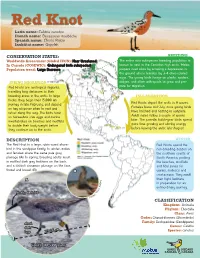
Red Knot Latin Name: Calidris Canutus French Name: Bécasseau Maubèche Spanish Name: Chorlo Rojizo Inuktitut Name: Qajorlak
Red Knot Latin name: Calidris canutus French name: Bécasseau maubèche Spanish name: Chorlo Rojizo Inuktitut name: Qajorlak CONSERVATION STATUS: BREEDING Worldwide Assessment (Global IUCN): Near threatened The entire rufa subspecies breeding population is In Canada (COSEWIC): Endangered (rufa subspecies) known to nest in the Canadian high arctic. Males Population trend: Large Decrease prepare nest sites by scraping a depression in the ground where females lay 3-4 olive-colored eggs. The young birds forage on plants, spiders, SPRING MIGRATION midges, and other arthropods to grow and pre- Red Knots are neotropical migrants, pare for migration. travelling long distances to their breeding areas in the arctic. In large FALL MIGRATION flocks, they begin their 15,000 km journey in late February, and depend Red Knots depart the arctic in 3 waves. on key stopover sites to rest and Females leave mid-July, once young birds refuel along the way. The birds feast have hatched and nesting is complete. on horseshoe crab eggs and marine Adult males follow a couple of weeks invertebrates on beaches and mudflats later. The juvenile, hatch-year birds spend to double their body weight before more time growing and storing energy they continue on to the arctic. before leaving the arctic late-August. DESCRIPTION WINTER The Red Knot is a large, robin-sized shore- Red Knots spend the bird in the sandpiper family. In winter, males non-breeding season on and females share the same pale gray the southern coasts of plumage (A). In spring, breeding adults moult South America, probing in mottled dark gray feathers on the back, the beaches, mudflats and a distinct cinnamon plumage on the face, and tidal zones for throat and breast (B). -

Phylogeny, Transposable Element and Sex Chromosome Evolution of The
bioRxiv preprint doi: https://doi.org/10.1101/750109; this version posted August 28, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. Phylogeny, transposable element and sex chromosome evolution of the basal lineage of birds Zongji Wang1,2,3,* Jilin Zhang4,*, Xiaoman Xu1, Christopher Witt5, Yuan Deng3, Guangji Chen3, Guanliang Meng3, Shaohong Feng3, Tamas Szekely6,7, Guojie Zhang3,8,9,10,#, Qi Zhou1,2,11,# 1. MOE Laboratory of Biosystems Homeostasis & Protection, Life Sciences Institute, Zhejiang University, Hangzhou, 310058, China 2. Department of Molecular Evolution and Development, University of Vienna, Vienna, 1090, Austria 3. BGI-Shenzhen, Beishan Industrial Zone, Shenzhen 518083, China 4. Department of Medical Biochemistry and Biophysics, Karolinska Institutet, SE-171 77, Stockholm, Sweden 5. Department of Biology and the Museum of Southwestern Biology, University of New Mexico, Albuquerque, NM 87131, USA 6. State Key Laboratory of Biocontrol, Department of Ecology, School of Life Sciences, Sun Yat-sen University, Guangzhou, 510275, China 7. Milner Center for Evolution, Department of Biology and Biochemistry, University of Bath, Bath, BA1 7AY, UK 8 State Key Laboratory of Genetic Resources and Evolution, Kunming Institute of Zoology, Chinese Academy of Sciences, Kunming 650223, China 9. Section for Ecology and Evolution, Department of Biology, University of Copenhagen, DK-2100 Copenhagen, Denmark 10. Center for Excellence in Animal Evolution and Genetics, Chinese Academy of Sciences, Kunming, 650223, China 11. Center for Reproductive Medicine, The 2nd Affiliated Hospital, School of Medicine, Zhejiang University * These authors contributed equally to the work.