Proposal of New Clades Based on Robust Phylogenetic Hypotheses
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

MARINE MAMMALS, EXTINCTIONS of Glenn R
MARINE MAMMALS, EXTINCTIONS OF Glenn R. VanBlaricom,* Leah R. Gerber,† and Robert L. Brownell, Jr.‡ *U.S. Geological Survey and University of Washington, †University of California, Santa Barbara, and ‡National Marine Fisheries Service I. Introduction principal source for taxonomic nomenclature, includ- II. Patterns and Case Studies of Extinction in ing common names, is the recent review of Rice (1998). Marine Mammals The order Cetacea includes whales, dolphins, and III. Discussion porpoises (Table I). The ‘‘pinnipedia’’ is a group of species in three families in the mammalian order Carni- vora (Table I). The pinnipeds include the seals, fur seals, sea lions, and walrus. The term pinnipedia is no I. INTRODUCTION longer recognized formally by marine mammal taxono- mists, but it continues to appear in the systematic ver- A. Taxonomic Definition of nacular as a matter of tradition and convenience. The order Sirenia includes the extant manatees and dugong ‘‘Marine Mammals’’ and the extinct Steller’s sea cow (Table I). The order The marine mammals include one extinct order and Desmostylia is the only recognized order of marine three major extant taxa that were or are fully aquatic, mammals to become entirely extinct. in most cases occurring entirely in the marine habitats Two largely terrestrial families of the order Carnivora of the major ocean basins and associated coastal seas also include species recognized as marine mammals and estuaries. In addition, a few species of largely terres- (Table I). Sea otters and chungungos (family Mustel- trial taxa are currently regarded as marine mammals. idae) live entirely or primarily in marine habitats. Polar We consider 127 recent mammal species in total to bears (family Ursidae) also spend a significant propor- be marine mammals for purposes of this review. -

Download Full Article in PDF Format
A new marine vertebrate assemblage from the Late Neogene Purisima Formation in Central California, part II: Pinnipeds and Cetaceans Robert W. BOESSENECKER Department of Geology, University of Otago, 360 Leith Walk, P.O. Box 56, Dunedin, 9054 (New Zealand) and Department of Earth Sciences, Montana State University 200 Traphagen Hall, Bozeman, MT, 59715 (USA) and University of California Museum of Paleontology 1101 Valley Life Sciences Building, Berkeley, CA, 94720 (USA) [email protected] Boessenecker R. W. 2013. — A new marine vertebrate assemblage from the Late Neogene Purisima Formation in Central California, part II: Pinnipeds and Cetaceans. Geodiversitas 35 (4): 815-940. http://dx.doi.org/g2013n4a5 ABSTRACT e newly discovered Upper Miocene to Upper Pliocene San Gregorio assem- blage of the Purisima Formation in Central California has yielded a diverse collection of 34 marine vertebrate taxa, including eight sharks, two bony fish, three marine birds (described in a previous study), and 21 marine mammals. Pinnipeds include the walrus Dusignathus sp., cf. D. seftoni, the fur seal Cal- lorhinus sp., cf. C. gilmorei, and indeterminate otariid bones. Baleen whales include dwarf mysticetes (Herpetocetus bramblei Whitmore & Barnes, 2008, Herpetocetus sp.), two right whales (cf. Eubalaena sp. 1, cf. Eubalaena sp. 2), at least three balaenopterids (“Balaenoptera” cortesi “var.” portisi Sacco, 1890, cf. Balaenoptera, Balaenopteridae gen. et sp. indet.) and a new species of rorqual (Balaenoptera bertae n. sp.) that exhibits a number of derived features that place it within the genus Balaenoptera. is new species of Balaenoptera is relatively small (estimated 61 cm bizygomatic width) and exhibits a comparatively nar- row vertex, an obliquely (but precipitously) sloping frontal adjacent to vertex, anteriorly directed and short zygomatic processes, and squamosal creases. -

I I 71-15,061 CAMERON, Christopher Paul, 1940- PALEOMAGNETISM of SHEMYA and ADAK ISLANDS, ALEUTIAN ISLANDS, ALASKA. University O
Paleomagnetism Of Shemya And Adak Islands, Aleutian Islands, Alaska Item Type Thesis Authors Cameron, Christopher Paul Download date 23/09/2021 14:56:00 Link to Item http://hdl.handle.net/11122/9194 I I 71-15,061 CAMERON, Christopher Paul, 1940- PALEOMAGNETISM OF SHEMYA AND ADAK ISLANDS, ALEUTIAN ISLANDS, ALASKA. University of Alaska, Ph.D., 1970 Geology University Microfilms, A XEROX Company, Ann Arbor, Michigan tutc nTCCTDTATTOM MAC HTTM MTPROFIT.MFD F.VAPTT.Y AS RF.OF.TVF.D Reproduced with permission of the copyright owner. Further reproduction prohibited without permission. PALE01IAGNETISM OF SHEMYA AMD ADAK ISLAUDS, ALEUTIAN ISLANDS, ALASKA A DISSERTATION Presented to the Faculty of the University of Alaska in Partial Fulfillment of the Requirements for the Degree of DOCTOR OF PHILOSOPHY by Christopher P/" Cameron B. S. College, Alaska May, 1970 Reproduced with permission of the copyright owner. Further reproduction prohibited without permission. PALEOilAGNETISM OF SHEMYA AND ADAK ISLANDS, ALEUTIAN ISLANDS, ALASKA APPROVED: f t l ‘y l .V" ■i. n ■ ■< < ; N w 1 T *W -C ltc-JL It / _ _ ____ /vx... , ~ ~ 7 YdSV Chairman APPPvOVED: dai£ 3 / 3 0 / 7 0 Dean of the College of Earth Sciences and Mineral Industry Vice President for Research and Advanced Study Reproduced with permission of the copyright owner. Further reproduction prohibited without permission. ABSTRACT Paleomagnetic results are presented for Tertiary and Quaternary volcanic rocks from Shemya and Adak Islands, Aleutian Islands, Alaska. The specimens were collected and measured using standard paleomagnetic methods. Alternating field demagnetization techniques were applied to test the stability of the remanence and to remove unwanted secondary components of magnetization. -

Sirenian Feeding Apparatus: Functional Morphology of Feeding Involving Perioral Bristles and Associated Structures
THE SIRENIAN FEEDING APPARATUS: FUNCTIONAL MORPHOLOGY OF FEEDING INVOLVING PERIORAL BRISTLES AND ASSOCIATED STRUCTURES By CHRISTOPHER DOUGLAS MARSHALL A DISSERTATION PRESENTED TO THE GRADUATE SCHOOL OF THE UNrVERSITY OF FLORIDA IN PARTIAL FULFILLMENT OF THE REOUIREMENTS FOR THE DEGREE OF DOCTOR OF PHILOSOPHY UNIVERSITY OF FLORIDA 1997 DEDICATION to us simply as I dedicate this work to the memory of J. Rooker (known "Rooker") and to sirenian conservation. Rooker was a subject involved in the study during the 1993 sampling year at Lowry Park Zoological Gardens. Rooker died during the red tide event in May of 1996; approximately 140 other manatees also died. During his rehabilitation at Lowry Park Zoo, Rooker provided much information regarding the mechanism of manatee feeding and use of the perioral bristles. The "mortality incident" involving the red tide event in southwest Florida during the summer of 1996 should serve as a reminder that the Florida manatee population and the status of all sirenians is precarious. Although some estimates suggest that the Florida manatee population may be stable, annual mortality numbers as well as habitat degradation continue to increase. Sirenian conservation and research efforts must continue. ii ACKNOWLEDGMENTS Research involving Florida manatees required that I work with several different government agencies and private parks. The staff of the Sirenia Project, U.S. Geological Service, Biological Resources Division - Florida Caribbean Science Center has been most helpful in conducting the behavioral aspect of this research and allowed this work to occur under their permit (U.S. Fish and Wildlife Permit number PRT-791721). Numerous conversations regarding manatee biology with Dr. -

PDF of Manuscript and Figures
The triple origin of whales DAVID PETERS Independent Researcher 311 Collinsville Avenue, Collinsville, IL 62234, USA [email protected] 314-323-7776 July 13, 2018 RH: PETERS—TRIPLE ORIGIN OF WHALES Keywords: Cetacea, Mysticeti, Odontoceti, Phylogenetic analyis ABSTRACT—Workers presume the traditional whale clade, Cetacea, is monophyletic when they support a hypothesis of relationships for baleen whales (Mysticeti) rooted on stem members of the toothed whale clade (Odontoceti). Here a wider gamut phylogenetic analysis recovers Archaeoceti + Odontoceti far apart from Mysticeti and right whales apart from other mysticetes. The three whale clades had semi-aquatic ancestors with four limbs. The clade Odontoceti arises from a lineage that includes archaeocetids, pakicetids, tenrecs, elephant shrews and anagalids: all predators. The clade Mysticeti arises from a lineage that includes desmostylians, anthracobunids, cambaytheres, hippos and mesonychids: none predators. Right whales are derived from a sister to Desmostylus. Other mysticetes arise from a sister to the RBCM specimen attributed to Behemotops. Basal mysticetes include Caperea (for right whales) and Miocaperea (for all other mysticetes). Cetotheres are not related to aetiocetids. Whales and hippos are not related to artiodactyls. Rather the artiodactyl-type ankle found in basal archaeocetes is also found in the tenrec/odontocete clade. Former mesonychids, Sinonyx and Andrewsarchus, nest close to tenrecs. These are novel observations and hypotheses of mammal interrelationships based on morphology and a wide gamut taxon list that includes relevant taxa that prior studies ignored. Here some taxa are tested together for the first time, so they nest together for the first time. INTRODUCTION Marx and Fordyce (2015) reported the genesis of the baleen whale clade (Mysticeti) extended back to Zygorhiza, Physeter and other toothed whales (Archaeoceti + Odontoceti). -

71St Annual Meeting Society of Vertebrate Paleontology Paris Las Vegas Las Vegas, Nevada, USA November 2 – 5, 2011 SESSION CONCURRENT SESSION CONCURRENT
ISSN 1937-2809 online Journal of Supplement to the November 2011 Vertebrate Paleontology Vertebrate Society of Vertebrate Paleontology Society of Vertebrate 71st Annual Meeting Paleontology Society of Vertebrate Las Vegas Paris Nevada, USA Las Vegas, November 2 – 5, 2011 Program and Abstracts Society of Vertebrate Paleontology 71st Annual Meeting Program and Abstracts COMMITTEE MEETING ROOM POSTER SESSION/ CONCURRENT CONCURRENT SESSION EXHIBITS SESSION COMMITTEE MEETING ROOMS AUCTION EVENT REGISTRATION, CONCURRENT MERCHANDISE SESSION LOUNGE, EDUCATION & OUTREACH SPEAKER READY COMMITTEE MEETING POSTER SESSION ROOM ROOM SOCIETY OF VERTEBRATE PALEONTOLOGY ABSTRACTS OF PAPERS SEVENTY-FIRST ANNUAL MEETING PARIS LAS VEGAS HOTEL LAS VEGAS, NV, USA NOVEMBER 2–5, 2011 HOST COMMITTEE Stephen Rowland, Co-Chair; Aubrey Bonde, Co-Chair; Joshua Bonde; David Elliott; Lee Hall; Jerry Harris; Andrew Milner; Eric Roberts EXECUTIVE COMMITTEE Philip Currie, President; Blaire Van Valkenburgh, Past President; Catherine Forster, Vice President; Christopher Bell, Secretary; Ted Vlamis, Treasurer; Julia Clarke, Member at Large; Kristina Curry Rogers, Member at Large; Lars Werdelin, Member at Large SYMPOSIUM CONVENORS Roger B.J. Benson, Richard J. Butler, Nadia B. Fröbisch, Hans C.E. Larsson, Mark A. Loewen, Philip D. Mannion, Jim I. Mead, Eric M. Roberts, Scott D. Sampson, Eric D. Scott, Kathleen Springer PROGRAM COMMITTEE Jonathan Bloch, Co-Chair; Anjali Goswami, Co-Chair; Jason Anderson; Paul Barrett; Brian Beatty; Kerin Claeson; Kristina Curry Rogers; Ted Daeschler; David Evans; David Fox; Nadia B. Fröbisch; Christian Kammerer; Johannes Müller; Emily Rayfield; William Sanders; Bruce Shockey; Mary Silcox; Michelle Stocker; Rebecca Terry November 2011—PROGRAM AND ABSTRACTS 1 Members and Friends of the Society of Vertebrate Paleontology, The Host Committee cordially welcomes you to the 71st Annual Meeting of the Society of Vertebrate Paleontology in Las Vegas. -

How to Cite Complete Issue More Information About This Article
Boletín de la Sociedad Geológica Mexicana ISSN: 1405-3322 Sociedad Geológica Mexicana, A.C. Hernández Cisneros, Atzcalli Ehécatl; González Barba, Gerardo; Fordyce, Robert Ewan Oligocene cetaceans from Baja California Sur, Mexico Boletín de la Sociedad Geológica Mexicana, vol. 69, no. 1, January-April, 2017, pp. 149-173 Sociedad Geológica Mexicana, A.C. Available in: http://www.redalyc.org/articulo.oa?id=94350664007 How to cite Complete issue Scientific Information System Redalyc More information about this article Network of Scientific Journals from Latin America and the Caribbean, Spain and Portugal Journal's homepage in redalyc.org Project academic non-profit, developed under the open access initiative Boletín de la Sociedad Geológica Mexicana / 2017 / 149 Oligocene cetaceans from Baja California Sur, Mexico Atzcalli Ehécatl Hernández Cisneros, Gerardo González Barba, Robert Ewan Fordyce ABSTRACT Atzcalli Ehecatl Hernández Cisneros ABSTRACT RESUMEN [email protected] Museo de Historia Natural de la Universidad Autónoma de Baja California Sur, Univer- Baja California Sur has an import- Baja California Sur tiene un importante re- sidad Autónoma de Baja California Sur, ant Cenozoic marine fossil record gistro de fósiles marinos del Cenozoico que Carretera al Sur Km 5.5, Apartado Postal which includes diverse but poorly incluye los restos poco conocidos de cetáceos 19-B, C.P. 23080, La Paz, Baja California Sur, México. known Oligocene cetaceans from del Oligoceno de México. En este estudio Instituto Politécnico Nacional, Centro Inter- Mexico. Here we review the cetacean ofrecemos más detalles sobre estos fósiles de disciplinario de Ciencias Marinas (CICMAR), fossil record including new observa- cetáceos, incluyendo nuevas observaciones Av. Instituto Politécnico Nacional s/n, Col. -

Ore Bin / Oregon Geology Magazine / Journal
THE ORE.-BIN Volume XVI Vol. 16, No.1 THE ORE.-BIN 1 January 1954 Portland, Oregon STATE DEPARTKENT OF GEOLOGY AND KINERAL INDUSTRIES Head Ottice: 1069 State Ottioe Bldg., Portland 1, Oregon Telephone: Columbia 2161, Ext. 488 State Governing Board ~ Kason L. Bingham, Chairman, Portland R. E. Corooran Geologist Niel R. Allen Grants Pass Hollis K. Dole Geologist Austin Dunn Baker L. L. Hoagland Assayer & Chemist Ralph S. Kason Kining Engineer F. W. Libbey, Direotor T. C. Katthews Speotrosoopist Lenin Ramp Geologist K. L. Steere Geologist R. E. stewart Geologist F18ld Ottices 20" First Street, Baker 2'9 S.E. "H" street, Grants Pass N. S. Wagper, Field Geologist David J. White, Field Geologist ****************************** THE ASTORIA LANDSLIDES Erosion to most ot us means the slow, almost imperoeptible, wearing down ot the higher parts ot the earth's crust by running water, wind, or ice. Seemingly mountains remain the same height and stream valleys the same depth throughout the years. The only rapid changes in the landscape which are accepted as normal are the neat exoavations made when new roads, dams, or other man-made structures are constructed. It 1s not sur prising that special attention to the ohoioe of a toundation for an ordinary building is seldom given, tor our experience tells us that the durability ot the structure is infinitely less than that of the ground on whioh it is bull t. It oomes as a shock to us when exceptions to our everyday observations ocour. Suoh is the oase in the land sliding at Astoria, Oregon,at the mouth of the Columbia River. -

The Developing Renal, Reproductive, and Respiratory Systems of the African Elephant Suggest an Aquatic Ancestry
Proc. Natl. Acad. Sci. USA Vol. 96, pp. 5555–5558, May 1999 Developmental Biology, Evolution The developing renal, reproductive, and respiratory systems of the African elephant suggest an aquatic ancestry A. P. GAETH*†,R.V.SHORT‡, AND M. B. RENFREE* *Department of Zoology, University of Melbourne, Parkville, Victoria 3052, Australia; and ‡Department of Perinatal Medicine, University of Melbourne, The Royal Women’s Hospital, Carlton, Victoria 3053, Australia Communicated by Judith Kimble, University of Wisconsin, Madison, WI, March 18, 1999 (received for review December 28, 1998) ABSTRACT The early embryology of the elephant has never of the testes, and the precocious development of the trunk could been studied before. We have obtained a rare series of African all have been adaptations to an aquatic lifestyle. elephant (Loxodonta africana) embryos and fetuses ranging in weight from 0.04 to 18.5 g, estimated gestational ages 58–166 MATERIALS AND METHODS days (duration of gestation is '660 days). Nephrostomes, a We have been able to obtain some rare African elephant spec- feature of aquatic vertebrates, were found in the mesonephric imens consisting of one embryo (weight 0.04 g) and six fetuses kidneys at all stages of development whereas they have never (weight ranging from 0.79 to 18.5 g) that were collected from been recorded in the mesonephric kidneys of other viviparous adult females shot in the Kruger National Park, South Africa, mammals. The trunk was well developed even in the earliest between 1993 and 1995, as part of a culling operation to reduce fetus. The testes were intra-abdominal, and there was no evidence elephant numbers in the park. -
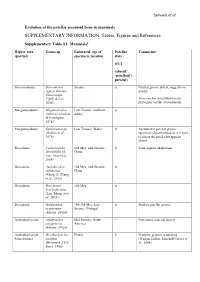
SUPPLEMENTARY INFORMATION: Tables, Figures and References
Samuels et al. Evolution of the patellar sesamoid bone in mammals SUPPLEMENTARY INFORMATION: Tables, Figures and References Supplementary Table S1: Mammals$ Higher taxa Genus sp. Estimated. age of Patellar Comments# (partial) specimen, location state 0/1/2 (absent/ ‘patelloid’/ present) Sinoconodonta Sinoconodon Jurassic 0 Patellar groove absent, suggests no rigneyi (Kielan- patella Jaworowska, Cifelli & Luo, Sinoconodon is included on our 2004) phylogeny within tritylodontids. Morganucodonta Megazostrodon Late Triassic, southern 0 rudnerae (Jenkins Africa & Parrington, 1976) Morganucodonta Eozostrodon sp. Late Triassic, Wales 0 Asymmetric patellar groove, (Jenkins et al., specimens disarticulated so it is hard 1976) to assess the patella but appears absent Docodonta Castorocauda 164 Mya, mid-Jurassic, 0 Semi-aquatic adaptations lutrasimilis (Ji, China Luo, Yuan et al., 2006) Docodonta Agilodocodon 164 Mya, mid-Jurassic, 0 scansorius China (Meng, Ji, Zhang et al., 2015) Docodonta Docofossor 160 Mya 0 brachydactylus (Luo, Meng, Ji et al., 2015) Docodonta Haldanodon 150-155 Mya, Late 0 Shallow patellar groove exspectatus Jurassic, Portugal (Martin, 2005b) Australosphenida Asfaltomylos Mid-Jurassic, South ? Postcranial material absent patagonicus America (Martin, 2005a) Australosphenida Ornithorhynchus Extant 2 Platypus, genome sequenced Monotremata anatinus (Warren, Hillier, Marshall Graves et (Herzmark, 1938; al., 2008) Rowe, 1988) Samuels et al. Australosphenida Tachyglossus + Extant 2 Echidnas Monotremata Zaglossus spp. (Herzmark, 1938; Rowe, 1988) Mammaliaformes Fruitafossor 150 Mya, Late Jurassic, 0 Phylogenetic status uncertain indet. windscheffeli (Luo Colorado & Wible, 2005) Mammaliaformes Volaticotherium Late Jurassic/Early ? Hindlimb material incomplete indet. antiquus (Meng, Cretaceous Hu, Wang et al., 2006) Eutriconodonta Jeholodens 120-125 Mya, Early 0 Poorly developed patellar groove jenkinsi (Ji, Luo Cretaceous, China & Ji, 1999) Eutriconodonta Gobiconodon spp. -

25 Squires and Fritsche
25 SQUIRES AND FRITSCHE Isurus sp. TRACE FOSSILS PI. 4, fig. 8 Unidentified burrows Isurus sp. (mako shark) teeth are the most " common shark teeth in the Sespe Creek area. Most The few unidentified burrows collected from the are moderately well preserved, nearly complete or Vaqueros Formation are about 9 cm long and about 1.5 complete, and not rounded by abrasion. Average cm in diameter. Burrows are more abundant in the length of complete specimens is about 1.5 cm; larger Santa Margarita Formation, where the most common type fragments are about 2.5 cm in length. is vertical, 1 to 3 cm in diameter, and straight to slightly curving. Some forms are branching, and at Genus Carcharodon Smith in Muller and Henle, 1838 locality 287, some resemble Ophiomorpha. Carcharodon angustidens L. Agassiz Kingdom PLANTAE PI. 4, fig. 3 Division RHODOPHYCOPHYTA Class RHODOPHYCEAE Carcharodon angustidens L. Agassiz, 1843, p. 255, Order CRYPTQNEMIALES pi. 28, figs. 20-25. Leriche, p. 13-14, pi. 11, figs. 8, 8a, 8b. Family Corallinaceae The figured specimen (PI. 4, fig. 3) of Carchar- Abundant whole and fragmented specimens of. an odon angustidens (great white shark) is a 6-cm-long unidentified coralline alga occur in a thin bed at tooth. Other specimens found include only a few locality 313. Small hemispherical colonies up to fragments. Remains of Carcharodon are previously 2.5 cm in height were collected, but most of the unreported from the Vaqueros Formation in the Sespe specimens are scattered fragments separated by Creek region (Loel and Corey, 1932). medium-grained sandstone. -

Early Eocene Fossils Suggest That the Mammalian Order Perissodactyla Originated in India
ARTICLE Received 7 Jul 2014 | Accepted 15 Oct 2014 | Published 20 Nov 2014 DOI: 10.1038/ncomms6570 Early Eocene fossils suggest that the mammalian order Perissodactyla originated in India Kenneth D. Rose1, Luke T. Holbrook2, Rajendra S. Rana3, Kishor Kumar4, Katrina E. Jones1, Heather E. Ahrens1, Pieter Missiaen5, Ashok Sahni6 & Thierry Smith7 Cambaytheres (Cambaytherium, Nakusia and Kalitherium) are recently discovered early Eocene placental mammals from the Indo–Pakistan region. They have been assigned to either Perissodactyla (the clade including horses, tapirs and rhinos, which is a member of the superorder Laurasiatheria) or Anthracobunidae, an obscure family that has been variously considered artiodactyls or perissodactyls, but most recently placed at the base of Proboscidea or of Tethytheria (Proboscidea þ Sirenia, superorder Afrotheria). Here we report new dental, cranial and postcranial fossils of Cambaytherium, from the Cambay Shale Formation, Gujarat, India (B54.5 Myr). These fossils demonstrate that cambaytheres occupy a pivotal position as the sister taxon of Perissodactyla, thereby providing insight on the phylogenetic and biogeographic origin of Perissodactyla. The presence of the sister group of perissodactyls in western India near or before the time of collision suggests that Perissodactyla may have originated on the Indian Plate during its final drift toward Asia. 1 Center for Functional Anatomy & Evolution, Johns Hopkins University School of Medicine, 1830 E. Monument Street, Baltimore, Maryland 21205, USA. 2 Department of Biological Sciences, Rowan University, Glassboro, New Jersey 08028, USA. 3 Department of Geology, H.N.B. Garhwal University, Srinagar 246175, Uttarakhand, India. 4 Wadia Institute of Himalayan Geology, Dehradun 248001, Uttarakhand, India. 5 Research Unit Palaeontology, Ghent University, Krijgslaan 281-S8, B-9000 Ghent, Belgium.