On the Hind Wing of Pseudolestes Mirabilis (Odonata: Pseudolestidae)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A Description of the Remarkable Larva of Pseudolestes Mirabilis Kirby (Odonata: Pseudolestidae)
International Journal of Odonatology Vol. 14, No. 2, June 2011, 105–110 A description of the remarkable larva of Pseudolestes mirabilis Kirby (Odonata: Pseudolestidae) Xin Yu* and Wenjun Bu Institute of Entomology, College of Life Sciences, Nankai University, Tianjin, 300071, PR China (Received 3 March 2011; final version received 26 May 2011) The larva of the Chinese endemic Pseudolestes mirabilis is described and figured for the first time. Speci- mens were collected from Hainan, the only known locality for this species. The presence of ventral paired gill tufts on S10 and sack-like caudal gills indicate that among other zygopteran families this species may be most closely related to the Amphipterygidae, but other characters, especially those of the adult suggest it may be sufficiently unique to warrant placement in its own family. Keywords: Odonata; dragonfly; Zygoptera; Pseudolestidae; Pseudolestes mirabilis; larva Introduction Pseudolestes mirabilis, the well-known Chinese endemic species, has been the subject of consid- erable recent speculation with regard to its phylogenetic position. Based on its unusual and unique adult characters the species was originally placed in its own subfamily by Kirby (1900). Calvert (1902) and Tillyard and Fraser (1938–1940) placed it in subfamily Lestinae (now Lestidae). Fraser (1957) then established the family Pseudolestidae to house Pseudolestes and several other genera, which was followed by Davies and Tobin (1984) and van Tol (2006). However recent phyloge- netic studies using molecular techniques (Bybee, Ogden, Branham & Whiting, 2008) suggest this species belongs in the Megapodagrionidae. The larvae of megapodagrionids have been divided into four groups by Kalkman, Choong, Orr and Schütte (2010): (1) species with inflated sack-like gills with a terminal filament; (2) species with flat vertical gills; (3) species in which the outer gills in life form a tube folded around the median gill; (4) species with flat horizontal gills. -

Odonata: Polythoridae) Melissa Sánchez-Herrera1,2* , Christopher D
Sánchez-Herrera et al. BMC Evolutionary Biology (2020) 20:74 https://doi.org/10.1186/s12862-020-01638-z RESEARCH ARTICLE Open Access An exploration of the complex biogeographical history of the Neotropical banner-wing damselflies (Odonata: Polythoridae) Melissa Sánchez-Herrera1,2* , Christopher D. Beatty3, Renato Nunes2,4, Camilo Salazar1 and Jessica L. Ware2,5 Abstract Background: The New World Tropics has experienced a dynamic landscape across evolutionary history and harbors a high diversity of flora and fauna. While there are some studies addressing diversification in Neotropical vertebrates and plants, there is still a lack of knowledge in arthropods. Here we examine temporal and spatial diversification patterns in the damselfly family Polythoridae, which comprises seven genera with a total of 58 species distributed across much of Central and South America. Results: Our time-calibrated phylogeny for 48 species suggests that this family radiated during the late Eocene (~ 33 Ma), diversifying during the Miocene. As with other neotropical groups, the Most Recent Common Ancestor (MRCA) of most of the Polythoridae genera has a primary origin in the Northern Andes though the MRCA of at least one genus may have appeared in the Amazon Basin. Our molecular clock suggests correlations with some major geographical events, and our biogeographical modeling (with BioGeoBEARS and RASP) found a significant influence of the formation of the Pebas and Acre systems on the early diversification of these damselflies, though evidence for the influence of the rise of the different Andean ranges was mixed. Diversification rates have been uniform in all genera except one—Polythore—where a significant increase in the late Pliocene (~ 3 mya) may have been influenced by recent Andean uplift. -

ESM-Table 1A/B. Species of the Suborders Anisoptera (A) and Zygoptera (B) Included in This Study; Ind
ESM-Table 1a/b. Species of the suborders Anisoptera (a) and Zygoptera (b) included in this study; Ind. = number of individuals analysed; ID = abbreviation of species name; Loc. = number of sample sites (localities). (a) Suborder: Anisoptera (b) Suborder: Zygoptera Family: Aeshnidae Family: Calopterygidae Species Ind. Loc. ID Species Ind. Loc. ID Aeshna cyanea 1 1 Aecy Phaon iridipennis 39 19 Pi Aeshna ellioti ellioti 1 1 Aelel Calopteryx haemorrhoidales 21 5 ch Aeshna ellioti usambarica 1 1 Aelus Calopteryx splendens 20 6 cs Aeshna grandis 1 1 Aegr Calopteryx virgo 51cv Aeshna rileyi 1 1 Aerl Coryphaeschna adnexa 1 1 Corad Family: Clorocyphidae Coryphaeschna perrensi 1 1 Corpe Anaciaeschna isosceles 1 1 Anaiso Chlorocypha aphrodite 1 1 Cap Anaciaeschna triangulifera 1 1 Anatri Platycypha amboniensis 21PA Anax imperator 88 16 Ai Platycypha auripes 2 1 Pau Anax junius 11Aj Platycypha caligata 56 11 Pc Anax parthenope 11Ap Anax speratus 21 4 As Family: Megapodagrionidae Anax ephippiger 19 4 Ae Brachytron pratense 1 1 Brpr Amanipodagrion gilliesi 11Ag Gynacantha manderica 1 1 Gyma Heteagrion sp. 2 1 Hsp Gynacantha usambarica 10 4 Gu Gynacantha villosa 1 1 Gyvill Family: Pseudolestidae Family: Gomphidae Rhipidolestes hiraoi 1 1 Rhd Paragomphus geneii 32 9 Pg Family: Coenagrionidae Family: Libellulidae Pseudagrion acaciae 42Pa Pseudagrion bicoerulans 22 4 Pb Nesciothemis farinosum 92Nf Pseudagrion commoniae 2 1 Pco Orthetrum brachiale 92Ob Pseudagrion gamblesi 2 1 Pga Orthetrum chrysostigma 34 9 Oc Pseudagrion hageni 21Ph Orthetrum coerulescens -

An Overview of Molecular Odonate Studies, and Our Evolutionary Understanding of Dragonfly and Damselfly (Insecta: Odonata) Behavior
International Journal of Odonatology Vol. 14, No. 2, June 2011, 137–147 Dragons fly, biologists classify: an overview of molecular odonate studies, and our evolutionary understanding of dragonfly and damselfly (Insecta: Odonata) behavior Elizabeth F. Ballare* and Jessica L. Ware Department of Biological Sciences, Rutgers, The State University of New Jersey, 195 University Ave., Boyden Hall, Newark, NJ, 07102, USA (Received 18 November 2010; final version received 3 April 2011) Among insects, perhaps the most appreciated are those that are esthetically pleasing: few capture the interest of the public as much as vibrantly colored dragonflies and damselflies (Insecta: Odonata). These remarkable insects are also extensively studied. Here, we review the history of odonate systematics, with an emphasis on discrepancies among studies. Over the past century, relationships among Odonata have been reinterpreted many times, using a variety of data from wing vein morphology to DNA. Despite years of study, there has been little consensus about odonate taxonomy. In this review, we compare odonate molecular phylogenetic studies with respect to gene and model selection, optimality criterion, and dataset completeness. These differences are discussed in relation to the evolution of dragonfly behavior. Keywords: Odonata; mitochondrion; nuclear; phylogeny; systematic; dragonfly; damselfly Introduction Why study Odonata? The order Odonata comprises three suborders: Anisozygoptera, Anisoptera, and Zygoptera. There are approximately 6000 species of Odonata described worldwide (Ardila-Garcia & Gregory, 2009). Of the three suborders Anisoptera and Zygoptera are by far the most commonly observed and collected, because there are only two known species of Anisozygoptera under the genus Epiophlebia. All odonate nymphs are aquatic, with a few rare exceptions such as the semi-aquatic Pseudocordulia (Watson, 1983), and adults are usually found near freshwater ponds, marshes, rivers (von Ellenrieder, 2010), streams, and lakes (although some species occur in areas of mild salinity; Corbet, 1999). -

Two Remarkable Fossil Insect Larvae from Burmese Amber Suggest the Presence of a Terminal Filum in the Direct Stem Lineage of Dragonflies and Damselflies (Odonata)
Rivista Italiana di Paleontologia e Stratigrafia (Research in Paleontology and Stratigraphy) vol. 126(1): 13-35. March 2020 TWO REMARKABLE FOSSIL INSECT LARVAE FROM BURMESE AMBER SUGGEST THE PRESENCE OF A TERMINAL FILUM IN THE DIRECT STEM LINEAGE OF DRAGONFLIES AND DAMSELFLIES (ODONATA) MARIO SCHÄDEL1*, PATRICK MÜLLER2 & JOACHIM T. HAUG1,3 1*Corresponding author. Department of Biology, Ludwig-Maximilians-Universität München, Großhaderner Str. 2, 82152 Planegg-Martinsried, Germany. E-mail: [email protected] 2Friedhofstr. 9, 66894 Käshofen, Germany. E-mail: [email protected] 3GeoBio-Center of the LMU Munich, Richard-Wagner-Str. 10, 80333 Munich, Germany. E-mail: [email protected] To cite this article: Schädel M., Müller P. & Haug J.T. (2020) - Two remarkable fossil insect larvae from Burmese amber suggest the presence of a terminal filum in the direct stem lineage of dragonflies and damselflies (Odonata). Riv. It. Paleontol. Strat., 126(1): 13-35. Keywords: character evolution; Cretaceous; moult; Myanmar; Odonatoptera; ontogeny. Abstract. The fossil record of dragonfly relatives (Odonatoptera) dates back to the Carboniferous, yet knowl- edge about these extinct animals is meagre. For most of the species little is known except for the characteristics of the wing venation. As a result, it is difficult to include fossil larvae in a (wing character based) phylogenetic tree as the wing venation is not visible in most of the larval instars. Two larval specimens from Cretaceous Burmese amber are in the focus of this study. The two specimens likely represent two subsequent early stage larval instars of the same individual. Not only is this an exceptional case to study ontogenetic processes in fossils – the larval instars are morphologically completely different from all known larvae of Odonata with respect to the posterior abdominal region. -

The Classification and Diversity of Dragonflies and Damselflies (Odonata)*
Zootaxa 3703 (1): 036–045 ISSN 1175-5326 (print edition) www.mapress.com/zootaxa/ Correspondence ZOOTAXA Copyright © 2013 Magnolia Press ISSN 1175-5334 (online edition) http://dx.doi.org/10.11646/zootaxa.3703.1.9 http://zoobank.org/urn:lsid:zoobank.org:pub:9F5D2E03-6ABE-4425-9713-99888C0C8690 The classification and diversity of dragonflies and damselflies (Odonata)* KLAAS-DOUWE B. DIJKSTRA1, GÜNTER BECHLY2, SETH M. BYBEE3, RORY A. DOW1, HENRI J. DUMONT4, GÜNTHER FLECK5, ROSSER W. GARRISON6, MATTI HÄMÄLÄINEN1, VINCENT J. KALKMAN1, HARUKI KARUBE7, MICHAEL L. MAY8, ALBERT G. ORR9, DENNIS R. PAULSON10, ANDREW C. REHN11, GÜNTHER THEISCHINGER12, JOHN W.H. TRUEMAN13, JAN VAN TOL1, NATALIA VON ELLENRIEDER6 & JESSICA WARE14 1Naturalis Biodiversity Centre, PO Box 9517, NL-2300 RA Leiden, The Netherlands. E-mail: [email protected]; [email protected]; [email protected]; [email protected]; [email protected] 2Staatliches Museum für Naturkunde Stuttgart, Rosenstein 1, 70191 Stuttgart, Germany. E-mail: [email protected] 3Department of Biology, Brigham Young University, 401 WIDB, Provo, UT. 84602 USA. E-mail: [email protected] 4Department of Biology, Ghent University, Ledeganckstraat 35, B-9000 Ghent, Belgium. E-mail: [email protected] 5France. E-mail: [email protected] 6Plant Pest Diagnostics Branch, California Department of Food & Agriculture, 3294 Meadowview Road, Sacramento, CA 95832- 1448, USA. E-mail: [email protected]; [email protected] 7Kanagawa Prefectural Museum of Natural History, 499 Iryuda, Odawara, Kanagawa, 250-0031 Japan. E-mail: [email protected] 8Department of Entomology, Rutgers University, Blake Hall, 93 Lipman Drive, New Brunswick, New Jersey 08901, USA. -

BIO-ECOLOGICAL STUDIES of MALAYSIAN ODONATES and an INTEGRATED TAXONOMIC STUDY on the GENUS Rhinocypha
BIO-ECOLOGICAL STUDIES OF MALAYSIAN ODONATES AND AN INTEGRATED TAXONOMIC STUDY ON THE GENUS Rhinocypha NOORHIDAYAH BINTI MAMATMalaya of FACULTY OF SCIENCE UNIVERSITY OF MALAYA UniversityKUALA LUMPUR 2018 BIO-ECOLOGICAL STUDIES OF MALAYSIAN ODONATES AND AN INTEGRATED TAXONOMIC STUDY ON THE GENUS Rhinocypha NOORHIDAYAH BINTI MAMAT Malaya THESIS SUBMITTED IN FULFILMENTof OF THE REQUIREMENT FOR THE DEGREE OF DOCTOR OF PHILOSOPHY INSTITUTE OF BIOLOGICAL SCIENCES FACULTY OF SCIENCE UNIVERSITY OF MALAYA UniversityKUALA LUMPUR 2018 UNIVERSITY OF MALAYA ORIGINAL LITERARY WORK DECLARATION Name of Candidate : NOORHIDAYAH BINTI MAMAT Matric No : SHC 140084 Name of Degree : DOCTOR OF PHILOSOPHY Title of Project Paper/Research Report/Dissertation/Thesis (“this Work”): BIO-ECOLOGICAL STUDIES OF MALAYSIAN ODONATES AND AN INTEGRATED TAXONOMIC STUDY ON THE GENUS Rhinocypha Field of Study: ECOLOGY & BIODIVERSITY (BIOLOGY & BIOCHEMISTRY) I do solemnly and sincerely declare that: (1) I am the sole author/writer of this Work; (2) This Work is original; (3) Any use of any work in which copyrightMalaya exists was done by way of fair dealing and for permitted purposes and any excerpt or extract from, or reference to or reproduction of any copyright work has been disclosed expressly and sufficiently and theof title of the Work and its authorship have been acknowledged in this Work; (4) I do not have any actual knowledge nor do I ought reasonably to know that the making of this work constitutes an infringement of any copyright work; (5) I hereby assign all and every rights in the copyright to this Work to the University of Malaya (“UM”), who henceforth shall be owner of the copyright in this Work and that any reproduction or use in any form or by any means whatsoever is prohibited without the written consent of UM having been first had and obtained; (6) I am fully aware that if in the course of making this Work I have infringed any copyright whether intentionally or otherwise, I may be subject to legal action or any other action as may be determined by UM. -

The Damselfly and Dragonfly Watercolour Collection of Edmond
International Journal of Odonatology, 2017 Vol. 20, No. 2, 79–112, https://doi.org/10.1080/13887890.2017.1330226 The damselfly and dragonfly watercolour collection of Edmond de Selys Longchamps: II Calopterygines, Cordulines, Gomphines and Aeschnines Karin Verspuia∗ and Marcel Th. Wasscherb aLingedijk 104, Tricht, the Netherlands; bMinstraat 15bis, Utrecht, the Netherlands (Received 3 March 2017; final version received 10 May 2017) In the nineteenth century Edmond de Selys Longchamps added watercolours, drawings and notes to his extensive collection of dragonfly and damselfly specimens. The majority of illustrations were exe- cuted by Selys and Guillaume Severin. The watercolour collection is currently part of the collection of the Royal Belgian Institute for Natural Sciences in Brussels. This previously unpublished material has now been scanned and is accessible on the website of this institute. This article presents the part of the collection concerning the following sous-familles according to Selys: Calopterygines (currently superfamilies Calopterygoidea and Epiophlebioidea), Cordulines (currently superfamily Libelluloidea), Gomphines (currently superfamily Petaluroidea, Gomphoidea, Cordulegastroidea and Aeshnoidea) and Aeschnines (currently superfamily Aeshnoidea). This part consists of 750 watercolours, 64 drawings and 285 text sheets. Characteristics and subject matter of the sheets with illustrations and text are pre- sented. The majority (92%) of all sheets with illustrations have been associated with current species names (Calopteryines 268, Cordulines 109, Gomphines 268 and Aeschnines 111). We hope the digital images and documentation stress the value of the watercolour collection of Selys and promote it as a source for odonate research. Keywords: Odonata; taxonomy; Severin; Zygoptera; Anisozygoptera; Anisoptera; watercolours; draw- ings; aquarelles Introduction The watercolour collection of Selys Edmond Michel de Selys Longchamps (1813–1900) did important work in odonate classifi- cation and taxonomy (Wasscher & Dumont, 2013; Verspui & Wasscher, 2016). -

Biogeography of Dragonflies and Damselflies: Highly Mobile Predators
14 Biogeography of Dragonflies and Damselflies: Highly Mobile Predators Melissa Sánchez-Herrera and Jessica L. Ware Department of Biology, Rutgers The State University of New Jersey, Newark Campus, USA 1. Introduction Dragonflies (Anisoptera) damselflies (Zygoptera) and Anisozygoptera comprise the three suborders of Odonata (“toothed ones”), often referred to as odonates. The Odonata are invaluable models for studies in ecology, behavior, evolutionary biology and biogeography and, along with mayflies (Ephemeroptera), make up the Palaeoptera, the basal-most group of winged insects. The Palaeoptera are thought to have diverged during the Jurassic (Grimaldi and Engel, 2005; Thomas et al., 2011), and as the basal-most pterygote group, odonates provide glimpses into the entomological past. Furthermore, few other insect groups possess as strong a fossil record as the Odonata and its precursors, the Protodonata, with numerous crown and stem group fossils from deposits worldwide. Their conspicuous behavior, striking colors and relatively small number of species (compared to other insect orders) has encouraged odonatological study. Odonates are important predators during both their larval and adult stages. They are often the top predators in freshwater ecosystems, such as rivers and lakes. One of their most remarkable traits, however, is their reproductive behavior, which takes place in a tandem position with the male and female engaging in a “copulatory wheel” (Fig. 1). Fig. 1. Odonata copulatory wheel (modified from Eva Paulson illustration Aug, 2010). 292 Global Advances in Biogeography During the last 5 decades, our understanding about the ecology and evolution of Odonata has increased dramatically (e.g., Cordoba-Aguilar, 2008). A fair odonate fossil record coupled with recent advances in molecular techniques, have inspired several biogeographical studies of Odonata. -

Remarks on the Taxonomy of Megapodagrionidae with Emphasis on the Larval Gills (Odonata)
Received 01 August 2009; revised and accepted 16 February 2010 Remarks on the taxonomy of Megapodagrionidae with emphasis on the larval gills (Odonata) 1 2 3 4 Vincent J. Kalkman , Chee Yen Choong , Albert G. Orr & Kai Schutte 1 National Museum of Natural History, P.O. Box 9517, 2300 RA Leiden, The Netherlands. <[email protected]> 2 Centre for Insect Systematics, Universiti Kebangsaan Malaysia, 43600 Bangi, Selangor, Malaysia. <[email protected]> 3 CRC, Tropical Ecosystem Management, AES, Griffith University, Nathan, Q4111, Australia. <[email protected]> 4 Biozentrum Grinde! und Zoologisches Museum, Martin-Luther-King-Piatz 3, 20146 Hamburg, Germany. <[email protected]> Key words: Odonata, dragonfly, Zygoptera, Megapodagrionidae, Pseudolestidae, taxonomy, larva. ABSTRACT A list of genera presently included in Megapodagrionidae and Pseudolestidae is pro vided, together with information on species for which the larva has been described. Based on the shape of the gills, the genera for which the larva is known can be ar ranged into four groups: (1) species with inflated sack-like gills with a terminal fila ment; (2) species with flat vertical gills; (3) species in which the outer gills in life form a tube folded around the median gill; (4) species with flat horizontal gills. The possible monophyly of these groups is discussed. It is noted that horizontal gills are not found in any other family of Zygoptera. Within the Megapodagrionidae the genera with horizontal gills are, with the exception of Dimeragrion, the only ones lacking setae on the shaft of the genital ligula. On the basis of these two characters it is suggested that this group is monophyletic. -

Agrion 25(2) - July 2021
Agrion 25(2) - July 2021 AGRION NEWSLETTER OF THE WORLDWIDE DRAGONFLY ASSOCIATION PATRON: Professor Edward O. Wilson FRS, FRSE Volume 25, Number 2 July 2021 Secretary and Treasurer: W. Peter Brown, Hill House, Flag Hill, Great Bentley, Colchester CO7 8RE. Email: wda.secretary@gmail. com. Editors: Keith D.P. Wilson. 18 Chatsworth Road, Brighton, BN1 5DB, UK. Email: [email protected]. Graham T. Reels. 31 St Anne’s Close, Badger Farm, Winchester, SO22 4LQ, Hants, UK. Email: [email protected]. ISSN 1476-2552 Agrion 25(2) - July 2021 AGRION NEWSLETTER OF THE WORLDWIDE DRAGONFLY ASSOCIATION AGRION is the Worldwide Dragonfly Association’s (WDA’s) newsletter, which is normally published twice a year in January and July. Occasionally a special issue may be produced, as was the case in May 2020 when a special issue was published in response to the ongoing Covid-19 pandemic. The WDA aims to advance public education and awareness by the promotion of the study and conservation of dragonflies (Odonata) and their natural habitats in all parts of the world. AGRION covers all aspects of WDA’s activities; it communicates facts and knowledge related to the study and conservation of dragonflies and is a forum for news and information exchange for members. AGRION is freely available for downloading from the WDA website at [https://worlddragonfly.org/about/agrion/]. WDA is a Registered Charity (Not-for-Profit Organization), Charity No. 1066039/0. A ‘pdf’ of the WDA’s Constitution and byelaws can be found at its website link at [https://worlddragonfly.org/about/]. ________________________________________________________________________________ Editor’s notes Keith Wilson [[email protected]] WDA Membership Membership signing up and renewal process is handled by WDA directly from the WDA website. -
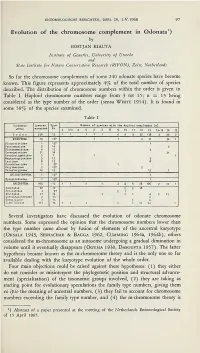
Evolution of the Chromosome Complement in Odonata1)
ENTOMOLOGISCHE BERICHTEN, DEEL 28, 1.V.1968 97 Evolution of the chromosome complement in Odonata1) by BOSTJAN KIAUTA Institute of Genetics, University of Utrecht and State Institute for Nature Conservation Research (RIVON), Zeist, Netherlands So far the chromosome complements of some 240 odonate species have become known. This figure represents approximately 4% of the total number of species described. The distribution of chromosome numbers within the order is given in Table I. Haploid chromosome numbers range from 3 tot 15; n z= 13 being considered as the type number of the order (sensu White 1954). It is found in some 58% of the species examined. Table I Taxonomic Species Type Number of species with the haploid complement (n) tjroup examined Ho 3 3-4 4 5 6 7 8 9 10 11 12 13 13-14 14 15 Order 236 13 11 111 4 4 5 30 138 2 46 2 ZYGOPTERA 70 13? 1 1 2 31 34 1 Platystictidae 1 13? 1 Protoneuridae 2 14 2 Platycnemididae 4 13 4 Coenagrionidae 31 ■ 14 31 Pseudosti^natidae 2 ? 1 1 Megapodagrionidae 5 13 5 Lestidae 8 13 8 Pseudolestidae 1 9? 1 Folythoridae 2 12? 2 Calopterygidae 14 13 13 1 AN ISOZ Y G0PT5RA 1 13? 1 Epiophlebiidae 1 13? 1 AKISOPTERA 165 13 1 1 1 1 3 4 5 28 106 2 12 1 Gomphidae 22 12 2 2 18 Petaluridae 3 9? 2 1 Aeshnidae 21 14 1 11 4 2 12 Cordulegasteridae 2 13 2 Coruuliiuae 7 13 1 6 Libellulidae 110 13 111 1 1 10 94 1 Several investigators have discussed the evolution of odonate chromosome numbers.