When Curiosity Saves the Cat Using Lures to Increase Effectiveness of Camera Traps When Studying Pallas’S Cat (Otocolobus Manul)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
VII. Bodies, Institutes and Centres
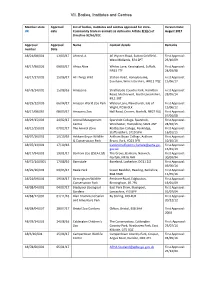
VII. Bodies, Institutes and Centres Member state Approval List of bodies, institutes and centres approved for intra- Version Date: UK date Community trade in animals as defined in Article 2(1)(c) of August 2017 Directive 92/65/EEC Approval Approval Name Contact details Remarks number Date AB/21/08/001 13/03/17 Ahmed, A 46 Wyvern Road, Sutton Coldfield, First Approval: West Midlands, B74 2PT 23/10/09 AB/17/98/026 09/03/17 Africa Alive Whites Lane, Kessingland, Suffolk, First Approval: NR33 7TF 24/03/98 AB/17/17/005 15/06/17 All Things Wild Station Road, Honeybourne, First Approval: Evesham, Worcestershire, WR11 7QZ 15/06/17 AB/78/14/002 15/08/16 Amazonia Strathclyde Country Park, Hamilton First Approval: Road, Motherwell, North Lanarkshire, 28/05/14 ML1 3RT AB/29/12/003 06/04/17 Amazon World Zoo Park Watery Lane, Newchurch, Isle of First Approval: Wight, PO36 0LX 15/06/12 AB/17/08/065 08/03/17 Amazona Zoo Hall Road, Cromer, Norfolk, NR27 9JG First Approval: 07/04/08 AB/29/15/003 24/02/17 Animal Management Sparsholt College, Sparsholt, First Approval: Centre Winchester, Hampshire, SO21 2NF 24/02/15 AB/12/15/001 07/02/17 The Animal Zone Rodbaston College, Penkridge, First Approval: Staffordshire, ST19 5PH 16/01/15 AB/07/16/001 10/10/16 Askham Bryan Wildlife Askham Bryan College, Askham First Approval: & Conservation Park Bryan, York, YO23 3FR 10/10/16 AB/07/13/001 17/10/16 [email protected]. First Approval: gov.uk 15/01/13 AB/17/94/001 19/01/17 Banham Zoo (ZSEA Ltd) The Grove, Banham, Norwich, First Approval: Norfolk, NR16 -
Carnivores of Syria 229 Doi: 10.3897/Zookeys.31.170 RESEARCH ARTICLE Launched to Accelerate Biodiversity Research
A peer-reviewed open-access journal ZooKeys 31: 229–252 (2009) Carnivores of Syria 229 doi: 10.3897/zookeys.31.170 RESEARCH ARTICLE www.pensoftonline.net/zookeys Launched to accelerate biodiversity research Carnivores of Syria Marco Masseti Department of Evolutionistic Biology “Leo Pardi” of the University of Florence, Italy Corresponding author: Marco Masseti (marco.masseti@unifi .it) Academic editors: E. Neubert, Z. Amr | Received 14 April 2009 | Accepted 29 July 2009 | Published 28 December 2009 Citation: Masseti, M (2009) Carnivores of Syria. In: Neubert E, Amr Z, Taiti S, Gümüs B (Eds) Animal Biodiversity in the Middle East. Proceedings of the First Middle Eastern Biodiversity Congress, Aqaba, Jordan, 20–23 October 2008. ZooKeys 31: 229–252. doi: 10.3897/zookeys.31.170 Abstract Th e aim of this research is to outline the local occurrence and recent distribution of carnivores in Syria (Syrian Arab Republic) in order to off er a starting point for future studies. The species of large dimensions, such as the Asiatic lion, the Caspian tiger, the Asiatic cheetah, and the Syrian brown bear, became extinct in historical times, the last leopard being reputed to have been killed in 1963 on the Alauwit Mountains (Al Nusyriain Mountains). Th e checklist of the extant Syrian carnivores amounts to 15 species, which are essentially referable to 4 canids, 5 mustelids, 4 felids – the sand cat having been reported only recently for the fi rst time – one hyaenid, and one herpestid. Th e occurrence of the Blandford fox has yet to be con- fi rmed. Th is paper is almost entirely the result of a series of fi eld surveys carried out by the author mainly between 1989 and 1995, integrated by data from several subsequent reports and sightings by other authors. -

ATIC0943 {By Email}
Animal and Plant Health Agency T 0208 2257636 Access to Information Team F 01932 357608 Weybourne Building Ground Floor Woodham Lane www.gov.uk/apha New Haw Addlestone Surrey KT15 3NB Our Ref: ATIC0943 {By Email} 4 October 2016 Dear PROVISION OF REQUESTED INFORMATION Thank you for your request for information about zoos which we received on 26 September 2016. Your request has been handled under the Freedom of Information Act 2000. The information you requested and our response is detailed below: “Please can you provide me with a full list of the names of all Zoos in the UK. Under the classification of 'Zoos' I am including any place where a member of the public can visit or observe captive animals: zoological parks, centres or gardens; aquariums, oceanariums or aquatic attractions; wildlife centres; butterfly farms; petting farms or petting zoos. “Please also provide me the date of when each zoo has received its license under the Zoo License act 1981.” See Appendix 1 for a list that APHA hold on current licensed zoos affected by the Zoo License Act 1981 in Great Britain (England, Scotland and Wales), as at 26 September 2016 (date of request). The information relating to Northern Ireland is not held by APHA. Any potential information maybe held with the Department of Agriculture, Environment and Rural Affairs Northern Ireland (DAERA-NI). Where there are blanks on the zoo license start date that means the information you have requested is not held by APHA. Please note that the Local Authorities’ Trading Standard departments are responsible for administering and issuing zoo licensing under the Zoo Licensing Act 1981. -

Pallas's Cat Status Review & Conservation
ISSN 1027-2992 I Special Issue I N° 13 | Spring 2019 Pallas'sCAT cat Status Reviewnews & Conservation Strategy 02 CATnews is the newsletter of the Cat Specialist Group, Editors: Christine & Urs Breitenmoser a component of the Species Survival Commission SSC of the Co�chairs IUCN/SSC International Union for Conservation of Nature (IUCN). It is pu���� Cat Specialist Group lished twice a year, and is availa�le to mem�ers and the Friends of KORA, Thunstrasse 31, 3074 Muri, the Cat Group. Switzerland Tel ++41(31) 951 90 20 For joining the Friends of the Cat Group please contact Fax ++41(31) 951 90 40 Christine Breitenmoser at [email protected] <urs.�[email protected]�e.ch> <ch.�[email protected]> Original contri�utions and short notes a�out wild cats are welcome Send contributions and observations to Associate Editors: Ta�ea Lanz [email protected]. Guidelines for authors are availa�le at www.catsg.org/catnews This Special Issue of CATnews has �een produced with Cover Photo: Camera trap picture of manul in the support from the Taiwan Council of Agriculture's Forestry Bureau, Kot�as Hills, Kazakhstan, 20. July 2016 Fondation Segré, AZA Felid TAG and Zoo Leipzig. (Photo A. Barashkova, I Smelansky, Si�ecocenter) Design: �ar�ara sur�er, werk’sdesign gm�h Layout: Ta�ea Lanz and Christine Breitenmoser Print: Stämpfli AG, Bern, Switzerland ISSN 1027-2992 © IUCN SSC Cat Specialist Group The designation of the geographical entities in this pu�lication, and the representation of the material, do not imply the expression of any opinion whatsoever on the part of the IUCN concerning the legal status of any country, territory, or area, or its authorities, or concerning the delimitation of its frontiers or �oundaries. -

Mammals of Jordan
© Biologiezentrum Linz/Austria; download unter www.biologiezentrum.at Mammals of Jordan Z. AMR, M. ABU BAKER & L. RIFAI Abstract: A total of 78 species of mammals belonging to seven orders (Insectivora, Chiroptera, Carni- vora, Hyracoidea, Artiodactyla, Lagomorpha and Rodentia) have been recorded from Jordan. Bats and rodents represent the highest diversity of recorded species. Notes on systematics and ecology for the re- corded species were given. Key words: Mammals, Jordan, ecology, systematics, zoogeography, arid environment. Introduction In this account we list the surviving mammals of Jordan, including some reintro- The mammalian diversity of Jordan is duced species. remarkable considering its location at the meeting point of three different faunal ele- Table 1: Summary to the mammalian taxa occurring ments; the African, Oriental and Palaearc- in Jordan tic. This diversity is a combination of these Order No. of Families No. of Species elements in addition to the occurrence of Insectivora 2 5 few endemic forms. Jordan's location result- Chiroptera 8 24 ed in a huge faunal diversity compared to Carnivora 5 16 the surrounding countries. It shelters a huge Hyracoidea >1 1 assembly of mammals of different zoogeo- Artiodactyla 2 5 graphical affinities. Most remarkably, Jordan Lagomorpha 1 1 represents biogeographic boundaries for the Rodentia 7 26 extreme distribution limit of several African Total 26 78 (e.g. Procavia capensis and Rousettus aegypti- acus) and Palaearctic mammals (e. g. Eri- Order Insectivora naceus concolor, Sciurus anomalus, Apodemus Order Insectivora contains the most mystacinus, Lutra lutra and Meles meles). primitive placental mammals. A pointed snout and a small brain case characterises Our knowledge on the diversity and members of this order. -

The Status and Distribution of Mediterranean Mammals
THE STATUS AND DISTRIBUTION OF MEDITERRANEAN MAMMALS Compiled by Helen J. Temple and Annabelle Cuttelod AN E AN R R E IT MED The IUCN Red List of Threatened Species™ – Regional Assessment THE STATUS AND DISTRIBUTION OF MEDITERRANEAN MAMMALS Compiled by Helen J. Temple and Annabelle Cuttelod The IUCN Red List of Threatened Species™ – Regional Assessment The designation of geographical entities in this book, and the presentation of material, do not imply the expression of any opinion whatsoever on the part of IUCN or other participating organizations, concerning the legal status of any country, territory, or area, or of its authorities, or concerning the delimitation of its frontiers or boundaries. The views expressed in this publication do not necessarily reflect those of IUCN or other participating organizations. Published by: IUCN, Gland, Switzerland and Cambridge, UK Copyright: © 2009 International Union for Conservation of Nature and Natural Resources Reproduction of this publication for educational or other non-commercial purposes is authorized without prior written permission from the copyright holder provided the source is fully acknowledged. Reproduction of this publication for resale or other commercial purposes is prohibited without prior written permission of the copyright holder. Red List logo: © 2008 Citation: Temple, H.J. and Cuttelod, A. (Compilers). 2009. The Status and Distribution of Mediterranean Mammals. Gland, Switzerland and Cambridge, UK : IUCN. vii+32pp. ISBN: 978-2-8317-1163-8 Cover design: Cambridge Publishers Cover photo: Iberian lynx Lynx pardinus © Antonio Rivas/P. Ex-situ Lince Ibérico All photographs used in this publication remain the property of the original copyright holder (see individual captions for details). -

Controlled Animals
Environment and Sustainable Resource Development Fish and Wildlife Policy Division Controlled Animals Wildlife Regulation, Schedule 5, Part 1-4: Controlled Animals Subject to the Wildlife Act, a person must not be in possession of a wildlife or controlled animal unless authorized by a permit to do so, the animal was lawfully acquired, was lawfully exported from a jurisdiction outside of Alberta and was lawfully imported into Alberta. NOTES: 1 Animals listed in this Schedule, as a general rule, are described in the left hand column by reference to common or descriptive names and in the right hand column by reference to scientific names. But, in the event of any conflict as to the kind of animals that are listed, a scientific name in the right hand column prevails over the corresponding common or descriptive name in the left hand column. 2 Also included in this Schedule is any animal that is the hybrid offspring resulting from the crossing, whether before or after the commencement of this Schedule, of 2 animals at least one of which is or was an animal of a kind that is a controlled animal by virtue of this Schedule. 3 This Schedule excludes all wildlife animals, and therefore if a wildlife animal would, but for this Note, be included in this Schedule, it is hereby excluded from being a controlled animal. Part 1 Mammals (Class Mammalia) 1. AMERICAN OPOSSUMS (Family Didelphidae) Virginia Opossum Didelphis virginiana 2. SHREWS (Family Soricidae) Long-tailed Shrews Genus Sorex Arboreal Brown-toothed Shrew Episoriculus macrurus North American Least Shrew Cryptotis parva Old World Water Shrews Genus Neomys Ussuri White-toothed Shrew Crocidura lasiura Greater White-toothed Shrew Crocidura russula Siberian Shrew Crocidura sibirica Piebald Shrew Diplomesodon pulchellum 3. -

Mammalian Predators Appropriating the Refugia of Their Prey
Mamm Res (2015) 60:285–292 DOI 10.1007/s13364-015-0236-y ORIGINAL PAPER When prey provide more than food: mammalian predators appropriating the refugia of their prey William J. Zielinski 1 Received: 30 September 2014 /Accepted: 20 July 2015 /Published online: 31 July 2015 # Mammal Research Institute, Polish Academy of Sciences, Białowieża, Poland (outside the USA) 2015 Abstract Some mammalian predators acquire both food and predators) may play disproportionately important roles in their shelter from their prey, by eating them and using the refugia communities. the prey construct. I searched the literature for examples of predators that exhibit this behavior and summarize their taxo- Keywords Predator–prey . Dens . Herbivore . Behavior . nomic affiliations, relative sizes, and distributions. I hypothe- Habitat . Resting . Foraging sized that size ratios of species involved in this dynamic would be near 1.0, and that most of these interactions would occur at intermediate and high latitudes. Seventeen species of Introduction Carnivorans exploited at least 23 species of herbivores as food and for their refugia. Most of them (76.4 %) were in the Mammals require food and most require shelter, either to pro- Mustelidae; several small species of canids and a few tect them from predators or from thermal stress. Carnivorous herpestids were exceptions. Surprisingly, the average mammals are unique in that they subsist on mobile food predator/prey weight ratio was 10.51, but few species of pred- sources which, particularly if these sources are vertebrates, ators were more than ten times the weight of the prey whose may build their own refuges to help regulate their body tem- refugia they exploit. -

Pallas's Cat Status Review & Conservation Strategy
ISSN 1027-2992 I Special Issue I N° 13 | Spring 2019 Pallas'sCAT cat Status Reviewnews & Conservation Strategy 02 CATnews is the newsletter of the Cat Specialist Group, Editors: Christine & Urs Breitenmoser a component of the Species Survival Commission SSC of the Co-chairs IUCN/SSC International Union for Conservation of Nature (IUCN). It is pub- Cat Specialist Group lished twice a year, and is available to members and the Friends of KORA, Thunstrasse 31, 3074 Muri, the Cat Group. Switzerland Tel ++41(31) 951 90 20 For joining the Friends of the Cat Group please contact Fax ++41(31) 951 90 40 Christine Breitenmoser at [email protected] <[email protected]> <[email protected]> Original contributions and short notes about wild cats are welcome Send contributions and observations to Associate Editors: Tabea Lanz [email protected]. Guidelines for authors are available at www.catsg.org/catnews This Special Issue of CATnews has been produced with Cover Photo: Camera trap picture of manul in the support from the Taiwan Council of Agriculture's Forestry Bureau, Kotbas Hills, Kazakhstan, 20. July 2016 Fondation Segré, AZA Felid TAG and Zoo Leipzig. (Photo A. Barashkova, I Smelansky, Sibecocenter) Design: barbara surber, werk’sdesign gmbh Layout: Tabea Lanz and Christine Breitenmoser Print: Stämpfli AG, Bern, Switzerland ISSN 1027-2992 © IUCN SSC Cat Specialist Group The designation of the geographical entities in this publication, and the representation of the material, do not imply the expression of any opinion whatsoever on the part of the IUCN concerning the legal status of any country, territory, or area, or its authorities, or concerning the delimitation of its frontiers or boundaries. -

World Distribution of the European Rabbit (Oryctolagus Cuniculus)
1 The Evolution, Domestication and World Distribution of the European Rabbit (Oryctolagus cuniculus) Luca Fontanesi1*, Valerio Joe Utzeri1 and Anisa Ribani1 1Department of Agricultural and Food Sciences, Division of Animal Sciences, University of Bologna, Italy 1.1 The Order Lagomorpha to assure essential vitamin uptake, the digestion of the vegetarian diet and water reintroduction The European rabbit (Oryctolagus cuniculus, (Hörnicke, 1981). Linnaeus 1758) is a mammal belonging to the The order Lagomorpha was recognized as a order Lagomorpha. distinct order within the class Mammalia in Lagomorphs are such a distinct group of 1912, separated from the order Rodentia within mammalian herbivores that the very word ‘lago- which lagomorphs were originally placed (Gidely, morph’ is a circular reference meaning ‘hare- 1912; Landry, 1999). Lagomorphs are, however, shaped’ (Chapman and Flux, 1990; Fontanesi considered to be closely related to the rodents et al., 2016). A unique anatomical feature that from which they diverged about 62–100 million characterizes lagomorphs is the presence of years ago (Mya), and together they constitute small peg-like teeth immediately behind the up- the clade Glires (Chuan-Kuei et al., 1987; Benton per-front incisors. For this feature, lagomorphs and Donoghue, 2007). Lagomorphs, rodents and are also known as Duplicidentata. Therefore, primates are placed in the major mammalian instead of four incisor teeth characteristic of clade of the Euarchontoglires (O’Leary et al., 2013). rodents (also known as Simplicidentata), lago- Modern lagomorphs might be evolved from morphs have six. The additional pair is reduced the ancestral lineage from which derived the in size. Another anatomical characteristic of the †Mimotonidae and †Eurymilydae sister taxa, animals of this order is the presence of an elong- following the Cretaceous-Paleogene (K-Pg) bound- ated rostrum of the skull, reinforced by a lattice- ary around 65 Mya (Averianov, 1994; Meng et al., work of bone, which is a fenestration to reduce 2003; Asher et al., 2005; López-Martínez, 2008). -

In Our Hands: the British and UKOT Species That Large Charitable Zoos & Aquariums Are Holding Back from Extinction (AICHI Target 12)
In our hands: The British and UKOT species that Large Charitable Zoos & Aquariums are holding back from extinction (AICHI target 12) We are: Clifton & West of England Zoological Society (Bristol Zoo, Wild Places) est. 1835 Durrell Wildlife Conservation Trust (Jersey Zoo) est. 1963 East Midland Zoological Society (Twycross Zoo) est. 1963 Marwell Wildlife (Marwell Zoo) est. 1972 North of England Zoological Society (Chester Zoo) est. 1931 Royal Zoological Society of Scotland (Edinburgh Zoo, Highland Wildlife Park) est. 1913 The Deep est. 2002 Wild Planet Trust (Paignton Zoo, Living Coasts, Newquay Zoo) est. 1923 Zoological Society of London (ZSL London Zoo, ZSL Whipsnade Zoo) est. 1826 1. Wildcat 2. Great sundew 3. Mountain chicken 4. Red-billed chough 5. Large heath butterfly 6. Bermuda skink 7. Corncrake 8. Strapwort 9. Sand lizard 10. Llangollen whitebeam 11. White-clawed crayfish 12. Agile frog 13. Field cricket 14. Greater Bermuda snail 15. Pine hoverfly 16. Hazel dormouse 17. Maiden pink 18. Chagos brain coral 19. European eel 2 Executive Summary: There are at least 76 species native to the UK, Crown Dependencies, and British Overseas Territories which Large Charitable Zoos & Aquariums are restoring. Of these: There are 20 animal species in the UK & Crown Dependencies which would face significant declines or extinction on a global, national, or local scale without the action of our Zoos. There are a further 9 animal species in the British Overseas Territories which would face significant declines or extinction without the action of our Zoos. These species are all listed as threatened on the IUCN Red List. There are at least 19 UK animal species where the expertise of our Zoological Institutions is being used to assist with species recovery. -

Appendix Lagomorph Species: Geographical Distribution and Conservation Status
Appendix Lagomorph Species: Geographical Distribution and Conservation Status PAULO C. ALVES1* AND KLAUS HACKLÄNDER2 Lagomorph taxonomy is traditionally controversy, and as a consequence the number of species varies according to different publications. Although this can be due to the conservative characteristic of some morphological and genetic traits, like general shape and number of chromosomes, the scarce knowledge on several species is probably the main reason for this controversy. Also, some species have been discovered only recently, and from others we miss any information since they have been first described (mainly in pikas). We struggled with this difficulty during the work on this book, and decide to include a list of lagomorph species (Table 1). As a reference, we used the recent list published by Hoffmann and Smith (2005) in the “Mammals of the world” (Wilson and Reeder, 2005). However, to make an updated list, we include some significant published data (Friedmann and Daly 2004) and the contribu- tions and comments of some lagomorph specialist, namely Andrew Smith, John Litvaitis, Terrence Robinson, Andrew Smith, Franz Suchentrunk, and from the Mexican lagomorph association, AMCELA. We also include sum- mary information about the geographical range of all species and the current IUCN conservation status. Inevitably, this list still contains some incorrect information. However, a permanently updated lagomorph list will be pro- vided via the World Lagomorph Society (www.worldlagomorphsociety.org). 1 CIBIO, Centro de Investigaça˜o em Biodiversidade e Recursos Genéticos and Faculdade de Ciˆencias, Universidade do Porto, Campus Agrário de Vaira˜o 4485-661 – Vaira˜o, Portugal 2 Institute of Wildlife Biology and Game Management, University of Natural Resources and Applied Life Sciences, Gregor-Mendel-Str.