Neochmia Phaeton Evangelinae
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

2007 Birds in a Changing Climate
T HE S TATE OF A USTRALIA ’ S B IRDS 2 0 0 7 Birds in a Changing Climate Compiled by Penny Olsen Supplement to Wingspan, vol. 14, no. 4, December 2007 2 The State of Australia’s Birds 2007 The State of Australia’s Birds 2007 3 The term climate change is commonly used to refer to shifts in modern climate, including the rise in average surface temperature known as global warming or the enhanced greenhouse effect. We use it here to refer to anthropogenic climate change, under the presumption that it is overwhelmingly caused by humans and hence has potential to largely be stabilised or reversed. The State of Australia’s Birds report series presents an overview of the status of the nation’s birds, the major threats they face and the conservation actions needed. This fifth annual report focuses on climate change. The climate is always changing and birds respond by adapting and evolving. However, at least since the very early 1900s the surface temperature of the earth has been warming at a rate unprecedented in human history, bringing with it shifts in local, regional and global weather systems. The vast majority of scientists agree that this is almost all attributable to the release of excessive amounts of greenhouse gases through human activity, particularly the use of fossil fuels and deforestation. Australia has warmed by 0.9 ° C since 1900, most rapidly since 1950, and is expected to warm a further 1 ° C over the next two decades. Signs of this change are already reflected in the distribution and abundance of some of our birds and in the timing of their breeding and migration, in line with changes observed in other biota. -

The Crimson Finch
PUBLISHED FOR BIRD LOVERS BY BIRD LOVERS life Aviarywww.aviarylife.com.au Issue 04/2015 $12.45 Incl. GST Australia The Red Strawberry Finch Crimson Finch Black-capped Lory One Week in Brazil The Red-breasted Goose ISSN 1832-3405 White-browed Woodswallow The Crimson Finch A Striking Little Aussie! Text by Glenn Johnson Photos by Julian Robinson www.flickr.com/photos/ozjulian/ Barbara Harris www.flickr.com/photos/12539790@N00/ Jon Irvine www.flickr.com/photos/33820263@N07/ and Aviarylife. Introduction he Crimson Finch Neochmia phaeton has Talways been one of the rarer Australian finches in captivity, and even more so since the white- the mid-late 1980’s, when the previously legal bellied. The trapping of wild finches in Australia was crown is dark prohibited across all states. They unfortunately brown, the back and have a bad reputation for being aggressive, wings are paler brown washed with red, the tail and this together with the fact that they is long, scarlet on top and black underneath. are reasonably expensive in comparison to The cheeks along with the entire under parts are many other finches, could well be a couple deep crimson, the flanks are spotted white, and of the main reasons as to why they are not so the centre of the belly is black in the nominate commonly kept. race and white for N. p. evangelinae, and the Description beak is red. Hens are duller, with black beaks. They are an elegant bird, generally standing There are two types of Crimson Finches, the very upright on the perch, and range from 120- black-bellied, which is the nominate form and 140mm in length. -

Volume 2. Animals
AC20 Doc. 8.5 Annex (English only/Seulement en anglais/Únicamente en inglés) REVIEW OF SIGNIFICANT TRADE ANALYSIS OF TRADE TRENDS WITH NOTES ON THE CONSERVATION STATUS OF SELECTED SPECIES Volume 2. Animals Prepared for the CITES Animals Committee, CITES Secretariat by the United Nations Environment Programme World Conservation Monitoring Centre JANUARY 2004 AC20 Doc. 8.5 – p. 3 Prepared and produced by: UNEP World Conservation Monitoring Centre, Cambridge, UK UNEP WORLD CONSERVATION MONITORING CENTRE (UNEP-WCMC) www.unep-wcmc.org The UNEP World Conservation Monitoring Centre is the biodiversity assessment and policy implementation arm of the United Nations Environment Programme, the world’s foremost intergovernmental environmental organisation. UNEP-WCMC aims to help decision-makers recognise the value of biodiversity to people everywhere, and to apply this knowledge to all that they do. The Centre’s challenge is to transform complex data into policy-relevant information, to build tools and systems for analysis and integration, and to support the needs of nations and the international community as they engage in joint programmes of action. UNEP-WCMC provides objective, scientifically rigorous products and services that include ecosystem assessments, support for implementation of environmental agreements, regional and global biodiversity information, research on threats and impacts, and development of future scenarios for the living world. Prepared for: The CITES Secretariat, Geneva A contribution to UNEP - The United Nations Environment Programme Printed by: UNEP World Conservation Monitoring Centre 219 Huntingdon Road, Cambridge CB3 0DL, UK © Copyright: UNEP World Conservation Monitoring Centre/CITES Secretariat The contents of this report do not necessarily reflect the views or policies of UNEP or contributory organisations. -

An Update of Wallacels Zoogeographic Regions of the World
REPORTS To examine the temporal profile of ChC produc- specification of a distinct, and probably the last, 3. G. A. Ascoli et al., Nat. Rev. Neurosci. 9, 557 (2008). tion and their correlation to laminar deployment, cohort in this lineage—the ChCs. 4. J. Szentágothai, M. A. Arbib, Neurosci. Res. Program Bull. 12, 305 (1974). we injected a single pulse of BrdU into pregnant A recent study demonstrated that progeni- CreER 5. P. Somogyi, Brain Res. 136, 345 (1977). Nkx2.1 ;Ai9 females at successive days be- tors below the ventral wall of the lateral ventricle 6. L. Sussel, O. Marin, S. Kimura, J. L. Rubenstein, tween E15 and P1 to label mitotic progenitors, (i.e., VGZ) of human infants give rise to a medial Development 126, 3359 (1999). each paired with a pulse of tamoxifen at E17 to migratory stream destined to the ventral mPFC 7. S. J. Butt et al., Neuron 59, 722 (2008). + 18 8. H. Taniguchi et al., Neuron 71, 995 (2011). label NKX2.1 cells (Fig. 3A). We first quanti- ( ). Despite species differences in the develop- 9. L. Madisen et al., Nat. Neurosci. 13, 133 (2010). fied the fraction of L2 ChCs (identified by mor- mental timing of corticogenesis, this study and 10. J. Szabadics et al., Science 311, 233 (2006). + phology) in mPFC that were also BrdU+. Although our findings raise the possibility that the NKX2.1 11. A. Woodruff, Q. Xu, S. A. Anderson, R. Yuste, Front. there was ChC production by E15, consistent progenitors in VGZ and their extended neurogenesis Neural Circuits 3, 15 (2009). -

Birdquest Australia (Western and Christmas
Chestnut-backed Button-quail in the north was a bonus, showing brilliantly for a long time – unheard of for this family (Andy Jensen) WESTERN AUSTRALIA 5/10 – 27 SEPTEMBER 2017 LEADER: ANDY JENSEN ASSISTANT: STUART PICKERING ! ! 1 BirdQuest Tour Report: Western Australia (including Christmas Island) 2017 www.birdquest-tours.com Western Shrike-tit was one of the many highlights in the southwest (Andy Jensen) Western Australia, if it were a country, would be the 10th largest in the world! The BirdQuest Western Australia (including Christmas Island) 2017 tour offered an unrivalled opportunity to cover a large portion of this area, as well as the offshore territory of Christmas Island (located closer to Indonesia than mainland Australia). Western Australia is a highly diverse region with a range of habitats. It has been shaped by the isolation caused by the surrounding deserts. This isolation has resulted in a richly diverse fauna, with a high degree of endemism. A must visit for any birder. This tour covered a wide range of the habitats Western Australia has to offer as is possible in three weeks, including the temperate Karri and Wandoo woodlands and mallee of the southwest, the coastal heathlands of the southcoast, dry scrub and extensive uncleared woodlands of the goldfields, coastal plains and mangroves around Broome, and the red-earth savannah habitats and tropical woodland of the Kimberley. The climate varied dramatically Conditions ranged from minus 1c in the Sterling Ranges where we were scraping ice off the windscreen, to nearly 40c in the Kimberley, where it was dust needing to be removed from the windscreen! We were fortunate with the weather – aside from a few minutes of drizzle as we staked out one of the skulkers in the Sterling Ranges, it remained dry the whole time. -

A Review of the Distribution, Status and Ecology of the Star Finch Neochmia Ruficauda in Queensland
AUSTRALIAN 278 BIRD WATCHER AUSTRALIAN BIRD WATCHER 1998, 17, 278-289 A Review of the Distribution, Status and Ecology of the Star Finch Neochmia ruficauda in Queensland by GLENN H.OLMES, P.O. Box 1246, Atherton, Queensland 4883 Summary The Star Finch Neochmia ruficauda has been recorded in 35-37 one-degree blocks in Queensland. Most records concern the Edward River, Princess Charlotte Bay and Rockharnpton districts. Viable populations are probably now restricted to Cape York Peninsula. Typical habitat comprises grasslands or grassy open woodlands, near permanent water or subject to regular inundation. Some sites support shrubby regrowth caused by the clearing of formerly unsuitable denser woodlands. Recorded food items are all seeds, of five grass species and one sedge. Precise nest records are few, but large numbers of juveniles have been observed during the last two decades at Aurukun, Pormpuraaw, Kowanyarna and Princess Charlotte Bay. Threatening processes are discussed; livestock grazing in riparian situations is considered the most deleterious. Introduction The distribution, status and ecology of the Star Finch Neochmia ruficauda in Queensland require urgent review. Endemic to northern and eastern Australia, its populations have declined in most regions. Available evidence suggests that the greatest contraction in its distribution has occurred in Queensland (e.g. Blakers et al. 1984). It is extinct in New South Wales, but its distribution there was only oflirnited extent (Holmes 1996). The Star Finch is protected stringently in Queensland because it is gazetted as Endangered under the Nature Conservation Act 1992. This categorisation takes due account of 'biological vulnerability, extent of current knowledge ... and management needs'. -

Invasive Toads Shift Predatorprey Densities in Animal Communities By
Ecology, 96(9), 2015, pp. 2544–2554 Ó 2015 by the Ecological Society of America Invasive toads shift predator–prey densities in animal communities by removing top predators 1,2,6,7 2 3 4 5 J. SEAN DOODY, REBEKAH SOANES, CHRISTINA M. CASTELLANO, DAVID RHIND, BRIAN GREEN, 4 6 COLIN R. MCHENRY, AND SIMON CLULOW 1Department of Ecology and Evolutionary Biology, 569 Dabney Hall, University of Tennessee, Knoxville, Tennessee 37996-1610 USA 2Department of Biological Sciences, Monash University, Clayton, Victoria 3800 Australia 3Utah’s Hogle Zoo, 2600 Sunnyside Avenue, Salt Lake City, Utah 84108 USA 4Department of Anatomy and Developmental Biology, Monash University, Clayton, Victoria 3800 Australia 5Institute for Applied Ecology, University of Canberra, Australian Capital Territory 2601 Australia 6School of Environmental and Life Sciences, University of Newcastle, Callaghan, New South Wales 2308 Australia Abstract. Although invasive species can have substantial impacts on animal communities, cases of invasive species facilitating native species by removing their predators have rarely been demonstrated across vertebrate trophic linkages. The predictable spread of the invasive cane toad (Rhinella marina), however, offered a unique opportunity to quantify cascading effects. In northern Australia, three species of predatory monitor lizards suffered severe population declines due to toad-induced lethal toxic ingestion (yellow-spotted monitor [Varanus panoptes], Mertens’ water monitor [V. mertensi], Mitchell’s water monitor [V. mitchelli]). We, thus, predicted subsequent increases in the abundance and recruitment of prey species due to the reduction of those predators. Toad-induced population-level declines in the water monitor species approached 50% over a five-year period spanning the toad invasion, apparently causing fledging success of the Crimson Finch (Neochmia phaeton) to increase from 55% to 81%. -
NESTLING MOUTH Marklngs It '" "' of OLD WORLD FINCHES ESTLLU MIMICRY and COEVOLUTION of NESTING
NESTLING MOUTH MARklNGS It '" "' OF OLD WORLD FINCHES ESTLLU MIMICRY AND COEVOLUTION OF NESTING r - .. ;.-; 5.i A&+.FINCHES .-. '4 AND THEIR VIDUA BROOD PARASITES - . , , . :.. - i ' -, ,' $*.$$>&.--: 7 -.: ',"L dt$=%>df;$..;,4;x.;b,?b;.:, ;.:. -, ! ,I Vt .., . k., . .,.-. , .is: 8, :. BY ERT B. PAYNE MISCELLANEOUS PUBLICATIONS MUSEUM OF ZOOLOGY, UNIVERSITY OF MICHIGAN, NO. 194 Ann ntwi day, 2005 lSSN 0076-8405 PUBLICATIONS OF THE MUSEUM OF ZOOLOGY, UNIVERSITY OF MICHIGAN NO. 194 J. B. BLJR(.H,Editor JI.:NNIFERFBLMLEE, Assistcint Editor The publications of the Museum of Zoology, The University of Michigan, consist primarily of two series-the Mi.scel/aneous Pziblications and the Occa.siona1 Papers. Both series were founded by Dr. Bryant Walker, Mr. Bradshaw H. Swales, and Dr. W.W. Newcomb. Occasionally thc Museum publishes contributions outside of these series; beginning in 1990 thcsc arc titled Special Publications and arc numbered. All submitted manuscripts to any of the Museum's publications receive external review. The Occasional Papers, begun in 1913, serve as a medium for original studies based principally upon the collections in the Museum. They arc issued separately. When a sufficient number of pages has been printed to make a volume, a title page, table of contents, and an index are supplied to libraries and individuals on the mailing list for the series. The Miscellaneotls Pt~hlication.~,initiated in 1916, include monographic studies, papers on field and museum techniques, and other contributions not within the scope of the Occasional Papers, and are published separately. It is not intended that they be grouped into volurnes. Each number has a title page and, when necessary, a table of contents. -

Western Australia
WESTERN AUSTRALIA 16 AUGUST – 7 SPETEMBER 2003 TOUR REPORT LEADER: CHRIS DOUGHTY. Seven years of drought in Australia has finally ended, unfortunately, the drought ended when the group arrived in Albany where we experienced heavy rain and gale force winds. Although we left the rain behind in Albany, the strong winds persisted throughout the whole tour, making the birding more difficult. After a leisurely first afternoon recovering from our long-haul flights, we began our birding the next morning at Lake Monger in the pleasant suburbs of Perth. The large concentrations of waterbirds included a few pairs of the uncommon Blue-billed Duck, several bizarre Musk Ducks, including a male bird, which put on an equally bizarre courtship display, and rather more surprisingly, a small party of Short-billed Black-Cockatoos, a south-western endemic which does not normally occur in downtown Perth. Here we also encountered our first honeyeaters, including the striking White-cheeked Honeyeater. As we drove on south towards Narrogin, we came across another of the south-western endemics, a splendid, full-plumaged, male Western Rosella, perched obligingly in a dead tree by the roadside. In the afternoon, a visit to Dryandra State Forest produced three more south-western endemics: Red-capped Parrot, Rufous Treecreeper and Western Yellow Robin. However, the afternoon’s show was undoubtedly stolen by a couple of seriously endangered Numbats, which gave superb views as they foraged busily on the forest floor only metres from the bus. This once widespread marsupial ‘ground squirrel’ is now confined to Dryandra State Forest. We also enjoyed great looks at a couple of obliging Short- beaked Echidnas, with a supporting cast of several Western Grey Kangaroos. -

Tropical & Far North Queensland 9 Day Birding Tour
Bellbird Tours Pty Ltd PO Box 2008, BERRI SA 5343 AUSTRALIA Ph. 1800-BIRDING Ph. +61409 763172 www.bellbirdtours.com [email protected] Tropical & Far North Queensland 9 day birding tour Tropical Far North Queensland is without doubt one of known for such as Magnificent Riflebird, Frill-necked Monarch, Australia‟s top birding destinations. A variety of tropical Monarch, Blue-faced Parrot-finch, Lovely Fairy-wren, White- habitats including rainforests, palm-fringed beaches, White-streaked Honeyeater, Papuan Frogmouth, nesting Red mangrove-lined mudflats, savannahs and cool mountain Red Goshawks, Palm Cockatoo, Eclectus Parrot, Yellow-bellied ranges result in a bird diversity unparalleled elsewhere in bellied Kingfisher and many more, including of course the rare the country. rare Southern Cassowary. We‟ll have rare access to Golden- Commencing in Cairns, we‟ll bird the local area and head shouldered Parrot nesting sites; explore Lakefield National to well-known Kingfisher Park on the Atherton Tablelands, Park; we‟ll go night spotting for owls and mammals‟; and we‟ll then traverse the unique Cape York Peninsula. During this we‟ll spend two days in Iron Range National Park, one of tour we aim to find all Tropical Far North Queensland Australia‟s most important ecosystems. We‟ll also look for specialties. Expect over 200 species including all the mammals such as Bandicoot, Sugar Glider, Northern Striped endemics and other important birds this area is well- Striped Possum, Red-necked Wallaby and Tree Kangaroo. Read on below for the full -
Eton Range Realignment Project ATTACHMENT 2 to EPBC Ref: 2015/7552 Preliminary Documentation Residual Impact Assessment and Offset Proposal - 37
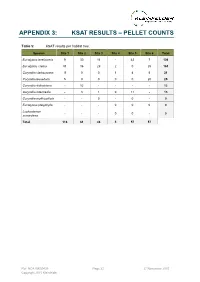
APPENDIX 3: KSAT RESULTS – PELLET COUNTS Table 5: KSAT results per habitat tree. Species Site 1 Site 2 Site 3 Site 4 Site 5 Site 6 Total Eucalyptus tereticornis 9 30 16 - 42 7 104 Eucalyptus crebra 91 16 29 2 0 25 163 Corymbia clarksoniana 11 0 0 1 4 5 21 Corymbia tessellaris 5 0 0 0 0 20 25 Corymbia dallachiana - 12 - - - - 12 Corymbia intermedia - 3 1 0 11 - 15 Corymbia erythrophloia - - 0 - 0 - 0 Eucalyptus platyphylla - - - 0 0 0 0 Lophostemon - - - 0 0 - 0 suaveolens Total 116 61 46 3 57 57 Ref: NCA15R30439 Page 22 27 November 2015 Copyright 2015 Kleinfelder APPENDIX 4: SITE PHOTOS The following images were taken from the centre of each BioCondition quadrat and represent a north east south west aspect, top left to bottom right. Ref: NCA15R30439 Page 23 27 November 2015 Copyright 2015 Kleinfelder Plate 3: BioCondition quadrat 1 (RE11.3.4/11.12.3) Ref: NCA15R30439 Page 24 27 November 2015 Copyright 2015 Kleinfelder Plate 4: BioCondition quadrat 2 (RE11.3.4/11.12.3) Ref: NCA15R30439 Page 25 27 November 2015 Copyright 2015 Kleinfelder Plate 5: BioCondition quadrat 3 (RE11.12.3) Ref: NCA15R30439 Page 26 27 November 2015 Copyright 2015 Kleinfelder Plate 6: BioCondition quadrat 4 (RE11.3.9) Ref: NCA15R30439 Page 27 27 November 2015 Copyright 2015 Kleinfelder Plate 7: BioCondition quadrat 5 (RE11.3.25) Ref: NCA15R30439 Page 28 27 November 2015 Copyright 2015 Kleinfelder Plate 8: BioCondition quadrat 6 (RE11.12.3/11.3.4/11.3.9) Ref: NCA15R30439 Page 29 27 November 2015 Copyright 2015 Kleinfelder Appendix E: Desktop Assessment for Potential -

EAZA Best Practice Guidelines for Turacos (Musophagidae)
EAZA BEST PRACTICE GUIDELINES EAZA Toucan & Turaco TAG TURACOS Musophagidae 1st Edition Compiled by Louise Peat 2017 1 | P a g e Front cover; Lady Ross’s chick. Photograph copyright of Eric Isselée-Life on White, taken at Mulhouse zoo. http://www.lifeonwhite.com/ http://www.zoo-mulhouse.com/ Author: Louise Peat. Cotswold Wildlife Park Email: [email protected] Name of TAG: Toucan & Turaco TAG TAG Chair: Laura Gardner E-mail: [email protected] 2 | P a g e EAZA Best Practice Guidelines disclaimer Copyright 2017 by EAZA Executive Office, Amsterdam. All rights reserved. No part of this publication may be reproduced in hard copy, machine-readable or other forms without advance written permission from the European Association of Zoos and Aquaria (EAZA). Members of the European Association of Zoos and Aquaria (EAZA) may copy this information for their own use as needed. The information contained in these EAZA Best Practice Guidelines has been obtained from numerous sources believed to be reliable. EAZA and the EAZA Toucan & Turaco TAG make a diligent effort to provide a complete and accurate representation of the data in its reports, publications, and services. However, EAZA does not guarantee the accuracy, adequacy, or completeness of any information. EAZA disclaims all liability for errors or omissions that may exist and shall not be liable for any incidental, consequential, or other damages (whether resulting from negligence or otherwise) including, without limitation, exemplary damages or lost profits arising out of or in connection with the use of this publication. Because the technical information provided in the EAZA Best Practice Guidelines can easily be misread or misinterpreted unless properly analysed, EAZA strongly recommends that users of this information consult with the editors in all matters related to data analysis and interpretation.