Diffusion-Controlled Spherulite Growth in Obsidian Inferred from H2O Concentration Profiles
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Spherulitic Aphyric Pillow-Lobe Metatholeiitic Dacite Lava of the Timmins Area, Ontario, Canada: a New Archean Facies Formed from Superheated Melts
©2008 Society of Economic Geologists, Inc. Economic Geology, v. 103, pp. 1365–1378 Spherulitic Aphyric Pillow-Lobe Metatholeiitic Dacite Lava of the Timmins Area, Ontario, Canada: A New Archean Facies Formed from Superheated Melts E. DINEL, B. M. SAUMUR, AND A. D. FOWLER,† Department of Earth Sciences, University of Ottawa, 140 Louis Pasteur, Ottawa, Canada K1N 6N5 and Ottawa Carleton Geoscience Centre Abstract Fragmental rocks of the V10 units of the Vipond Formation of the Tisdale assemblage previously have been identified as pillow basalts, but many samples are shown to be intermediate-to-felsic in character, likely tholei- itic dacite in composition. Specifically, the V10b unit is mapped as a pillow-lobe dacite. Aside from being more geochemically evolved in terms of their “immobile” trace elements, these rocks differ from typical pillow basalts in that they have more abundant primary breccia and hyaloclastite. The pillow lobes are contorted, hav- ing been folded in a plastic state and are zoned, typically having a spherulite-rich core. Moreover, the flows are aphyric, interpreted to mean that they were erupted in a superheated state. This along with their pillow-lobe nature demonstrates that they were erupted as relatively low-viscosity melts for such silicic compositions. In- teraction with water quenched the outer pillow lobe and contributed to the formation of the abundant brec- cia. The fact that the melt was crystal and microlite free inhibited crystal growth, such that the bulk of the lobes were quenched to crystal-free glass. Nucleation occurred only in the cores, where cooling rates were lower in comparison to the medial and exterior areas of the pillow lobes, although in the cores crystal growth rates were high so that abundant spherulite formation took place. -

Spherulite Formation in Obsidian Lavas in the Aeolian Islands, Italy
1 Spherulite formation in obsidian lavas in the Aeolian Islands, Italy 2 Running title: Spherulites in obsidian lavas, Aeolian Islands 3 4 Liam A. Bullocka, b*, Ralf Gertissera, Brian O’Driscolla, c 5 6 a) School of Geography, Geology and the Environment, Keele University, Keele, ST5 5BG, UK 7 b) Dept. of Geology & Petroleum Geology, Meston Building, University of Aberdeen, King’s College, 8 Aberdeen, AB24 3UE, UK 9 c) School of Earth and Environmental Science, University of Manchester, Williamson Building, Oxford 10 Road, Manchester, M13 9PL, UK 11 12 *Corresponding author. 13 Email [email protected] 14 15 16 ABSTRACT 17 Spherulites in obsidian lavas of Lipari and Vulcano (Italy) are characterised by spatial, textural and geochemical 18 variations, formed by different processes across flow extrusion and emplacement. Spherulites vary in size from 19 <1 mm to 8 mm, are spherical to elongate in shape, and show variable radial interiors. Spherulites occur 20 individually or in deformation bands, and some are surrounded by clear haloes and brown rims. Spherulites 21 typically contain cristobalite (α, β) and orthoclase, titanomagnetite and rhyolitic glass, and grew over an average 22 period of 5 days. 23 Heterogeneity relates to formation processes of spherulite ‘types’ at different stages of cooling and 24 emplacement. Distinct populations concentrate within deformation structures or in areas of low shear, with 25 variations in shape and internal structure. CSD plots show differing size populations and growth periods. 26 Spherulites which formed at high temperatures show high degree of elongation, where deformation may have 27 triggered formation. Spherulites formed at mid-glass transition temperatures are spherical, and all spherulites are 28 modified at vapour-phase temperatures. -

Spherulite Crystallization Induces Fe-Redox Redistribution in Silicic Melt Jonathan M
Spherulite crystallization induces Fe-redox redistribution in silicic melt Jonathan M. Castro, Elizabeth Cottrell, H. Tuffen, Amelia V. Logan, Katherine A. Kelley To cite this version: Jonathan M. Castro, Elizabeth Cottrell, H. Tuffen, Amelia V. Logan, Katherine A. Kelley. Spherulite crystallization induces Fe-redox redistribution in silicic melt. Chemical Geology, Elsevier, 2009, 268 (3-4), pp.272-280. 10.1016/j.chemgeo.2009.09.006. insu-00442797 HAL Id: insu-00442797 https://hal-insu.archives-ouvertes.fr/insu-00442797 Submitted on 23 Dec 2009 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Spherulite crystallization induces Fe-redox redistribution in silicic melt Jonathan M. Castro a, Elizabeth Cottrellb, Hugh Tuffenc, Amelia V. Loganb and Katherine A. Kelleyc, d aISTO, UMR 6113 Université d'Orléans-CNRS, 1a rue de la Férollerie, 45071 Orléans cedex 2, France bDepartment of Mineral Sciences, Smithsonian Institution, 10th and Constitution Ave. NW, Washington, DC 20560, USA cDepartment of Environmental Science, Lancaster University, LA1 4YQ, UK dGraduate School of Oceanography, University of Rhode Island, Narragansett, RI 02882, USA Abstract Rhyolitic obsidians from Krafla volcano, Iceland, record the interaction between mobile hydrous species liberated during crystal growth and the reduction of ferric iron in the silicate melt. -

Diffusion-Controlled Spherulite Growth in Obsidian Inferred from H2O Concentration Profiles
Contrib Mineral Petrol DOI 10.1007/s00410-008-0327-8 ORIGINAL PAPER Diffusion-controlled spherulite growth in obsidian inferred from H2O concentration profiles Jim Watkins Æ Michael Manga Æ Christian Huber Æ Michael Martin Received: 3 November 2007 / Accepted: 8 July 2008 Ó Springer-Verlag 2008 Abstract Spherulites are spherical clusters of radiating Keywords Spherulites Á Obsidian Á FTIR Á crystals that occur naturally in rhyolitic obsidian. The Advection–diffusion growth of spherulites requires diffusion and uptake of crystal forming components from the host rhyolite melt or glass, and rejection of non-crystal forming components Introduction from the crystallizing region. Water concentration profiles measured by synchrotron-source Fourier transform spec- Spherulites are polycrystalline solids that develop under troscopy reveal that water is expelled into the surrounding highly non-equilibrium conditions in liquids (Keith and matrix during spherulite growth, and that it diffuses out- Padden 1963). Natural spherulites are commonly found in ward ahead of the advancing crystalline front. We compare rhyolitic obsidian and have evoked the curiosity of petrol- these profiles to models of water diffusion in rhyolite to ogists for more than a century (e.g., Judd 1888; Cross 1891). estimate timescales for spherulite growth. Using a diffu- Over the past several decades, numerous studies on spher- sion-controlled growth law, we find that spherulites can ulite morphology (Keith and Padden 1964a; Lofgren grow on the order of days to months at temperatures above 1971a), kinetics of spherulite growth (Keith and Padden the glass transition. The diffusion-controlled growth law 1964b; Lofgren 1971b), disequilibrium crystal growth rates also accounts for spherulite size distribution, spherulite and textures (Fenn 1977; Swanson 1977), and field obser- growth below the glass transition, and why spherulitic vations (Mittwede 1988; Swanson et al. -
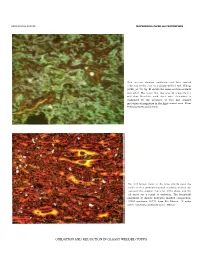
Ash-Flow Tuffs: Their Origin, Geologic Relations and Identification and Zones and Zonal Variations in Welded Ash Flows
GEOLOGICAL SURVEY PROFESSIONAL PAPER 366 FRONTISPIECE Thin section showing oxidation and later partial reduction of the iron in a glassy welded tuff. lddings (1899, pl. 50, fig. B) shows the same section in black and white. His belief that this was all originally red and that bleaching took place after deposition is confirmed by the presence of very fine grained microlites of magnetite in the light-colored area. From Yellowstone National Park. The dull brown cores of the large shards near the middle of this photomicrograph of glassy welded tuff represent the original character of the glass, and the red areas are a result of oxidation. The horizontal alinement of shards indicates marked compaction. USNM specimen 38771 from Rio Blanco, 10 miles north-northwest of Guadalajara, Mexico. OXIDATION AND REDUCTION IN GLASSY WELDED TUFFS Ash-Flow Tuffs: Their Origin, Geologic Relations and Identification and Zones and Zonal Variations in Welded Ash Flows with Foreword b y ROBERT L. SMITH Reprinted from U.S. Geological Survey Professional Papers 354-F and 366 Managing Editor JONATHAN F. CALLENDER NEWMEXICOGEOLOGICALSOCIETY SPECIAL PUBLICATION NO. 9 1980 CONTENTS Publications of New Mexico Geological Society ................................................................. Inside Front Cover Foreword .....................................................................................................................Robert L. Smith iii Preface .................................................................................................................James -

Some Little-Known Scenic Pleasure Places in the Cascade Range in Oregon by IRA A
.. VOLUME 2 NUMBER 1 '/ MAY, 1916 THE MINERAL RESOURCES OF ORE·GON Published M~nthly By The Oregon Bureau of Mines and Geology See Capitalize Oregon I Oregon First Scenery Waterfall in Cascade Range Photo by Weister Some Little-Known Scenic Pleasure Places in the Cascade Range in Oregon By IRA A. WILLIAMS 114 Pages ,66 Illustrations Entered as second cl""s matter at Corvallis, Ore. on Feb. 10, 1914, according to the Act of Aug. 24, 1912. OREGON BUREAU OF MINES AND GEOLOGY COMMISSION OniCE ON THE CoMMISSION AND EXHIBIT OREGON BUILDING, PORTLAND, OREGON OniCE o• THE DIRECTOR CORVALLIS, OREGOl-1 JAHES WITHYCOHBE, Governor HENRY M. PARKS, Director COMMISSION ABTHUR M. SwARTLEY, Mining Engineer H. N. LAWRIE, Portland IRA A. WILLIAMS, Ceramist W. C. FELLOWS, Sumpter 1. F .• REnnr, Medford 1. L. Woon, Albany R. M. BETTS, CO\"nucopia P. L. CAMPBELL, Eugene W. 1. KERR, Corvallis Volume 2 Number 1 May Issue of the MINERAL RESOURCES OF OREGON Published by ,.. The Oregon Bureau of Mines and Geology I • CONTAINING Some Little-Known Scenic Pleasure Places in the 1 Cascade Range in Oregon By IRA A. WILLIAMS l . 114 Pages 66 Illustrations 1916 ANNOUNCEMENT With this issue we present the first number of Volume.2 of The Mineral Resources of Ore gon. This is the first issue since December, 1914, and the first to be completed for publica tion giving results of field work during the past season. It is a preliminary paper involving the general geology of the Cascade Range and is to be followed by detailed reports upon the various other economic resources of the Range. -

Chiricahua National Monument Geologic Resources Inventory Report
National Park Service U.S. Department of the Interior Natural Resource Program Center Chiricahua National Monument Geologic Resources Inventory Report Natural Resource Report NPS/NRPC/GRD/NRR—2009/081 THIS PAGE: Close up of the many rhyolitic hoodoos within the Monument ON THE COVER: Scenic vista in Chiricahua NM NPS Photos by: Ron Kerbo Chiricahua National Monument Geologic Resources Inventory Report Natural Resource Report NPS/NRPC/GRD/NRR—2009/081 Geologic Resources Division Natural Resource Program Center P.O. Box 25287 Denver, Colorado 80225 June 2009 U.S. Department of the Interior National Park Service Natural Resource Program Center Denver, Colorado The Natural Resource Publication series addresses natural resource topics that are of interest and applicability to a broad readership in the National Park Service and to others in the management of natural resources, including the scientific community, the public, and the NPS conservation and environmental constituencies. Manuscripts are peer-reviewed to ensure that the information is scientifically credible, technically accurate, appropriately written for the intended audience, and is designed and published in a professional manner. Natural Resource Reports are the designated medium for disseminating high priority, current natural resource management information with managerial application. The series targets a general, diverse audience, and may contain NPS policy considerations or address sensitive issues of management applicability. Examples of the diverse array of reports published in this series include vital signs monitoring plans; "how to" resource management papers; proceedings of resource management workshops or conferences; annual reports of resource programs or divisions of the Natural Resource Program Center; resource action plans; fact sheets; and regularly-published newsletters. -

The Geology of the Tuff of Bridge Spring: Southern Nevada and Northwestern Arizona
UNLV Theses, Dissertations, Professional Papers, and Capstones 12-1993 The geology of the Tuff of Bridge Spring: Southern Nevada and northwestern Arizona Shirley Ann Morikawa University of Nevada, Las Vegas Follow this and additional works at: https://digitalscholarship.unlv.edu/thesesdissertations Part of the Geochemistry Commons, Geology Commons, Stratigraphy Commons, and the Volcanology Commons Repository Citation Morikawa, Shirley Ann, "The geology of the Tuff of Bridge Spring: Southern Nevada and northwestern Arizona" (1993). UNLV Theses, Dissertations, Professional Papers, and Capstones. 1453. The Geology of the TufT of Bridge Spring: Southern Nevada and Northwestern Arizona by Shirley Ann Morikawa A thesis submitted in partial fulfillment of the requirements for the degree of Master of Science in Geology Geoscience Department University of Nevada, Las Vegas December, 1993 © 1994 Shirley Ann Morikawa All Rights Reserved The thesis of Shirley Ann Morikawa for the degree of Master of Science in Geology is approve . r, Ernest M. Duebendorfer, Ph.D. Graduate Faculty Representaf , Donna E.Weistrop, Ph.D. Dean of the Graduate College, Ronald W. Smith, Ph.D. University of Nevada, Las Vegas December, 1993 ii Abstract The Tuff of Bridge Spring (TBS) is a regionally-widespread, andesite to rhyolite (59 .50 to 74.91 wt. %) ash-flow tuff of mid-Miocene age (ca. 15.2 Ma) that is exposed in the northern Colorado River extensional corridor of southern Nevada and northwestern Arizona. Determination of the areal distribution, geochronology, lithology, geochemistry, and internal stratigraphy of the TBS is important for its establishment as a reliable stratigraphic reference horizon for tectonic reconstructions of the extensional corridor during the middle Miocene. -

A Study of the Structure of Fulgurites
A STUDY OF THE STRUCTURE OF FULGURITES by ALEXIS A. JULIEN ORIGINALLY PUBLISHED IN JOURNAL OF GEOLOGY VOL. 9, 1901 ORIGINALLY PUBLISHED IN JOURNAL OF GEOLOGY VOL. 9, 1901 A STUDY OF THE STRUCTURE OF FULGURITES by ALEXIS A. JULIEN Some obscure problems in structures occurring within arti- ficial and natural glasses (rhyolites, tachylites, etc.) receive light from the study of fulgurites, as representatives of instantaneous fusion, and frequently, as it will be shown, of equally rapid de- vitrification. Hitherto, however, a constitution of homogeneous glass has been universally observed, under the microscope, in all sand fulgurites, without the least trace of devitrification in the larg- est masses.1 Occasional cloudy stains of brown iron-oxide and black manganese-oxide have been noted, and frequent enclo- 1“A Perfect Glass,” ARAGO (Ann. d. Ch. et. d. Phys., Vol. XIX (1821), p. 290) and all later investigators. sure of remnants of quartz-grains2 and of bubbles, both more abundant near the outer walls of fulgurite.3 In rock-fulgurites, a single instance of devitrification has been recorded. The re- sults of examination of four fulgurites will now be described. I. Fulgurite (lightning-tube) from sand, Poland; a small frag- ment, together with thin cross-sections, prepared by Mr. James Walker, of the New York Microscopical Society. This fulgurite is of small size, from 5 to 8mm in diameter, with central aper- ture or lumen usually 2.5 to 4mm in diameter, and glass wall varying mostly from 0.6 to 2.0mm in thickness, roughened outwardly by adhering sand-grains in a continuous coating. -

ERUPTIONAL and POST-ERUPTIONAL PROCESSES in RHYOLITE DOMES Volume 2: Drafts of Papers
ERUPTIONAL AND POST-ERUPTIONAL PROCESSES IN RHYOLITE DOMES Volume 2: Drafts of papers A THESIS SUBMITTED IN FULFILMENT OF THE REQUIREMENTS FOR THE DEGREE OF Doctor of Philosophy in Geological Sciences IN THE UNIVERSITY OF CANTERBURY by J ens Richnow University of Canterbury March 2000 1/ I' "1 I I \' I j(' / j ; ! Chapter 14 Discussion 14.1 Introduction In the review of the thesis by the examiners it was suggested that the summary given on pages 351-355 does not reflect the important results of the thesis in a generic manner. Therefore an additional synthesis chapter was required which interprets the results of the main findings in terms of testing a hypothesis (or hypotheses). This chapter is written in the form of drafts of potential papers, which stress important findings in the study. I would like to thank the examiners for suggesting this approach for two reasons: (i) it allows me to present results in a way more accessible to readers and (ii) it prepares and eases the publishing of these findings as papers. ! 1 - 7 APR 2000 14.2. DRAFT OF PAPER 1 14.2 Draft of paper 1: "Relationship between Cooling, Development and Distribu tion of Textures and Lithologies in Rhyolite Domes" Abstract Detailed studies of the distribution and significance of textural/lithological units in rhyolite domes and flows provide valuable insight into cooling, degassing and crystallisation processes during and after their emplacement. In this paper I investigate the relationship between the main lithologies present in rhyolite domes, morphological dome parameters such as dome height and radius, and the cooling history. -

REVISION 2 Nucleation Rates of Spherulites in Natural Rhyolitic Lava
1 REVISION 2 2 Nucleation Rates of Spherulites in Natural Rhyolitic Lava 3 4 5 James E. Gardner1,*, Kenneth S. Befus2, James M. Watkins3, and Travis Clow1 6 7 8 9 1Department of Geological Sciences, Jackson School of Geosciences, 10 The University of Texas at Austin, Austin, TX, 78712-0254, U.S.A. 11 2Department of Geosciences, Baylor University, Waco, TX, 76798, U.S.A. 12 3Department of Geological Sciences, University of Oregon, 13 Eugene, OR, 97403-1272, U.S.A. 14 15 16 17 18 *Author to whom correspondence should be addressed 19 (fax: 1-512-471-9425; office: 1-512-471-0953 20 e-mail: [email protected]) 21 22 ABSTRACT 23 The rates of nucleation and crystal growth from silicate melt are difficult to measure 24 because the temperature–time path of magma is often unknown. We use geochemical 25 gradients around spherulites in obsidian glass to estimate the temperature–time interval of 26 spherulite crystallization. This information is used in conjunction with new high- 27 resolution X-ray Computed Tomography (HRXCT) data on the size distributions of 28 spherulites in six samples of rhyolite obsidian lava to infer spherulite nucleation rates. A 29 large dataset of geochemical profiles indicate that the lavas cooled at rates of 10-2.2 to 10- 30 1.2 °C hr-1, and that the spherulites grew at rates that decreased exponentially with time, 31 with values of 10-0.70 to 100.30 µm hr-1 at 600°C. Spherulites are estimated to have begun 32 nucleating when undercooling [ΔT, = liquidus T (≈800°C) minus nucleation T] reached 33 100–277°C, and stopped when ΔT = 203–365°C, with exact values dependent on 34 assumed cooling and growth rates. -

The Island Park Caldera of Eastern Idaho
Geology and Petrogenesis of the Island Park Caldera of Rhyolite and Basalt Eastern Idaho GEOLOGICAL SURVEY PROFESSIONAL PAPER 504-C Geology and Petrogenesis of the Island Park Caldera of Rhyolite and Basalt Eastern Idaho By WARREN HAMILTON SHORTER CONTRIBUTIONS TO GENERAL GEOLOGY GEOLOGICAL SURVEY PROFESSIONAL PAPER 504-C A study of the origin and occurrence of the products of eruption and collapse of a large magma chamber in which liquid rhyolite overlay liquid basalt UNITED STATES GOVERNMENT PRINTING OFFICE, WASHINGTON : 1965 UNITED STATES DEPARTMENT OF THE INTERIOR STEWART L. UDALL, Secretary GEOLOGICAL SURVEY Thomas B. Nolan, Director For sa1e by the Superintendent of Documents, U.S. Government Printing Office Washington, D.C. 20402 CONTENTS Page Page Abstract------------------------------------------- Cl PetrologY------------------------------------------ C19 Introduction--------------------------------------- 1 PetrographY----------------------------------- 20 ~logy___________________________________________ 3 Rhyolite--------------------------------- 20 The caldera____________________________________ 4 Basalt------------------------------------- 20 Rhyolite of precaldera shield volcano__________ 4 Chemistry __ ----------------------------------- 21 Caldera rim fault _____ ---------------------- 6 Basalt_----------------------------------- 22 Rhyolite domes on caldera rim_______________ 8 Latite __ ----------------------------------- 24 Postcollapse rhyolite ash flows________________ 10 · Rhyolite-----------------------------------