Today for Tomorrow: Realizing the Potential of Life Sciences 4.0 Foreword
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
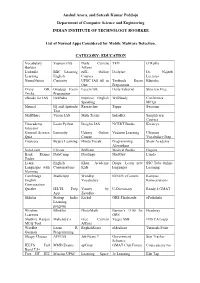
Anshul Arora, and Sateesh Kumar Peddoju Department of Computer Science and Engineering INDIAN INSTITUTE of TECHNOLOGY ROORKEE
Anshul Arora, and Sateesh Kumar Peddoju Department of Computer Science and Engineering INDIAN INSTITUTE OF TECHNOLOGY ROORKEE List of Normal Apps Considered for Mobile Malware Detection. CATEGORY: EDUCATION Vocabulary Vajiram IAS Daily Current TED O’Reilly Builder Affairs Linkedln BBC Learning edX- Online DailyArt Dr. Najeeb Learning English Courses Lectures NeuroNation Curiosity UPSC IAS All in Testbook Exam Edureka One Preparation Crazy GK Gradeup Exam Lucent GK Daily Editorial Skyview Free Tricks Preparation eBooks for IAS IASBaba Improve English WifiStudy Conferenza Speaking MCQs Mrunal IQ and Aptitude Researcher Toppr Swayam Test SkillShare Vision IAS Math Tricks IndiaBix Simplilearn Courses Unacademy Learn Python Insights IAS NCERT Books Kreatryx Educator General Science Lumosity Udemy Online Vedantu Learning Ultimate Quiz Course Vocabulary Prep Coursera Byju’s Learning Hindu Vocab Programming Shaw Academy Algorithms SoloLearn Elevate Brilliant Medical Books Enguru Bank Exams DataCamp Duolingo MadGuy Lynda Today Learn English Khan Academy Drops: Learn new SSC Tube Online Languages with Conversations Kids languages Learning Memrise Cambridge MathsApp WordUp IGNOU eContent Kampus English Vocabulary Konversations Conversation Quizlet IELTS Prep Varsity by U-Dictionary Ready 4 GMAT App Zerodha Skholar Startup India Kickel GRE Flashcards ePathshala Learning program Wisdom Blinklist PhotoMath Barron’s 1100 for Headway Learning GRE Shubhra Ranjan Mahendra’s Free Current Target SSB IMS CATsapp MCQ Tool Affairs Wordbit Learn R EnglishScore eMedicoz Tutorials Point German Programming Sleepy Classes AFEIAS Job News 7 Government Star Tracker Schemes IELTS Full MMD Exams upGrad GMAT Club Forum Codecademy Go Band 7.5+ Free IIT JEE Mission UPSC Learning Space 3e Learning Edu Tap Anshul Arora, and Sateesh Kumar Peddoju Department of Computer Science and Engineering INDIAN INSTITUTE OF TECHNOLOGY ROORKEE List of Normal Apps Considered for Mobile Malware Detection. -

Comments UHI Draft
To: National Health Authority, 9th Floor, Tower - 1 Jeevan Bharati Building, Connaught Place, New Delhi - 110001 23rd August 2021 We thank the National Health Authority for the opportunity to provide feedback on the Consultation Paper on Unified Health Interface. The process of consultation is a welcome one, and we hope that this spirit of transparency and due process is continued in the framing of future reports, policies by the Authority. We appreciate the Authority for reflecting feedback on the NDHM policy into this Consultation Paper (hereinafter referred as “paper”). We have been particularly pleased to see the approach to governance and stakeholder consultation adopted by this paper. However, we believe that this draft is still quite far away from a meaningful, implementable policy document - many of the ideas posited in the document, including the identification of stakeholders, pricing formula and mechanisms for grievance redressal, lack clarity and will lead to confusion and incorrect implementation. This is not to say that the conversation on the UHI is not important but in India where a personal data protection framework still does not exist, and sector specific data related regulations are being contemplated - the move to implement a UHI requires more public discussion. At Aapti Institute, we have been working on the idea of data stewardship and offline intermediaries, examining lived experiences at the intersection of technology and society. Our detailed submission below builds off our engagement on questions of agency, digital literacy and data rights, and draws from international best practices. We hope that this draft will go through several transparent iterations. -

Winning in the Indian Pharmaceutical Market
White Paper WINNING IN THE INDIAN PHARMACEUTICAL MARKET NOVEMBER 2018 TABLE OF CONTENTS Section 1: Exploring Domestic Archetypes Prevalent Business Archetypes in the Indian Pharmaceutical Market 5 Brand Builders 6 Therapy Leaders 7 Access Drivers 8 Specialty Players 9 Winning in the Indian Pharmaceutical Market 10 Evolution of the Archetypes 11 Section 2: Exploring MNC Archetypes Prevalent Business Archetypes for MNCs 13 India as the arrowhead 14 Therapy focused play 15 Patented play 16 Legacy- local play 17 Winning in the Indian Pharmaceutical Market for MNCs 18 Evolution of the Archetypes 21 Section 3: Alternate Channel Play and Investment Themes Alternate Channel Play 23 Hospital Channel 23 Trade Channel 25 e-Pharmacies 27 Importance of Channel Play 27 Investment Themes 28 Investment Themes in Healthcare 28 Investment Themes in Pharma 30 Drivers of PE Investments in Pharma 32 References 33 About the Authors 34 TABLEEXECUTIVE OF CONTENTS SUMMARY The Indian Pharmaceutical Market (IPM) is one of the fastest growing markets globally, growing at a rate of 10% per annum*, despite recent impact of demonetization and GST roll-out. Globally, IPM is the largest exporter of generics, by volume, with the total Indian pharma export market estimated at USD ~17 Billion,1 which is in addition to the domestic market of USD ~18 Billion.2 Furthermore, India accounts for ~40% of generic drug approvals in the US, based on FY17 data,3 thereby indicating increasing relevance of India in the global pharma market. It is pertinent to mention that over 10,000+ companies operate in India, employing 5,00,000+ people and providing medication to 1.3 Billion people.4,5 In recent times, increased pricing pressures in US market, has prompted Indian companies and the investor community to shift their focus to expanding and understanding the domestic business, respectively. -

New Startups in Healthcare System
© 2018 JETIR December 2018, Volume 5, Issue 12 www.jetir.org (ISSN-2349-5162) New Startups in Healthcare System Tushar Pandey, Ritu Sharma, Vivek Gupta* School of Pharmaceutical Sciences, Lovely Professional University, Phagwara, Punjab, 144411. Abstract: Startup is a new business in which people invest if it is a good and profitable idea. Nowadays many innovative ideas are becoming start-ups. The community not only wants to rely on traditional healthcare tricks and tips but want some innovation to smoothen the process. Healthcare is one of the sectors which are growing very fast and people are becoming aware of their fitness and health issues. So it is very much required for the industries to come up with new ideas and treatment which is not only safe but also effective. As we all know life is going fast and no one has time to go to doctor’s clinic due to their busy schedule but they also can't let their health go worse, therefore, such startups which are providing help to these kinds of patients are necessary. Online websites and the availability of such expertise with experience provide patients comfort and trust. It is a growing sector and to maintain their position in the market, industries need to come up with such good ideas that can not only save patient’s time and efforts but also proved helpful for the doctors. Introduction: Startup means starting a new work or process which is helpful for society [1]. If we are discussing something new in the field of healthcare let us talk about providing medical care to the people [1]. -

Online@Matrikiran Google Classrooms Contents
JAN – JUN 2020 Seamless shift to Online@MatriKiran Google Classrooms Contents New@Vatika Retail Shopper’s Village INXT, Gurgaon Town Square 2, Gurgaon Company Updates Market Walk, Gurgaon INXT Market Place 1 & 2, Gurgaon Vatika@Lockdown High Street At INXT, Gurgaon Ambala City Centre, Ambala Townships Education Possessions by Vatika MatriKiran School, Gurgaon Vatika India Next, Gurgaon Vatika India Next 2, Gurgaon Vatika Infotech City, Jaipur Vatika Interior Solutions Vatika City Central, Ambala Business Centres Commercial Vatika Mindscapes, Mathura Road One On One, Gurgaon Facilities Management INXT City Centre, Gurgaon MatriKiran students during their trip to Kechla, Odisha The Light House, Gurgaon VCare Jan – Jun 2020 New@Vatika Vatika Group has perfected the art of real estate through A mega retail centre, Public Square, has been launched at the 1.3 years of experience and earned a spot amongst the most 3 mn sq ft commercial campus – Vatika Mindscapes. Designed to prominent names of the industry. With an excellent track create a lively, vibrant and urbane atmosphere, Public Square will be record of design and delivery, many reputed organisations a home to prominent brands categories like Salons & Spas, Apparel have leased space in Vatika projects. Decathlon has Stores, F&B, Electronics, Hypermarket and many more. It is the only signed up at the upcoming retail destination, Shopper’s organised retail destination in Faridabad and shall cater to 38 lakh Village INXT. The company has also leased space people in the vicinity. It will also have a captive footfall of the 6,000+ to industry majors, Big Bazaar and Denstu. We are professionals working at Vatika Mindscapes. -

Vaccine Race
OPINION, P6 ECONOMY, P2 MARKETS, P10 T NANDA KUMAR EDITORIAL AMID FARMER PROTESTS REVIVAL MODE Mandatory MSP will TheAnilVij episode shows PM's pitch on reforms: SBI to accelerate awareness drives on vaccines hit the agri economy needed; ensure proper Cannot build new retail loans; sees at multiple fronts mask use and distancing century with old laws better earnings PUNE, TUESDAY, DECEMBER 8, 2020 FOLLOW US ON TWITTER & FACEBOOK. APP AVAILABLE ON APP STORE & PLAYSTORE WWW.FINANCIALEXPRESS.COM VOL. XI NO. 266, 14 PAGES,`6.00PUBLISHEDFROM:AHMEDABAD,BENGALURU,CHANDIGARH, CHENNAI,HYDERABAD,KOCHI,KOLKATA,LUCKNOW,MUMBAI, NEWDELHI,PUNE READ TO LEAD SENSEX: 45,426.97 ▲ 347.42 NIFTY: 13,355.75 ▲ 97.20 NIKKEI 225: 26,547.44 ▼ 203.80 HANG SENG: 26,506.85 ▼ 329.07 `/$: 73.90 ▼ 0.11 `/€: 89.36 ▲ 0.43 BRENT: $48.76 ▼ $0.49 GOLD: `49,089 ▼ `64 IN THE NEWS COVID COMBAT ● CENTRAL VISTA FLIGHT PLAN From Jan, quarterly SC allows GSTreturns to ease stone-laying Jet to take off in summer of 2021 Astra,Pfizervaccines: ■ RAGINI SAXENA Consortium led by Dubai- compliance burden Jet Airways (NSE) but no work December 7 80 based entrepreneur Murari Lal Jalan and Kalrock Capital has THE GOODS and services tax 71.45 (GST) payers with a turnover FE BUREAU BANKRUPT CARRIER Jet Air- set a revival plan for JetAirways of up to `5 crore will have Govt speeds up review New Delhi, December 7 ways India, once the nation’s 60 the option to ease their biggestairlinebymarketvalue, ■ ■ compliance burden from NEHAARORA & The Serum Serum THE SUPREME COURT on is planning to restart opera- 40 January 1, as the facility to NIVEDITABHATTACHARJEE vaccine is logis- Institute has Molnupiravir: Monday allowed the govern- tionsasafull-servicecarrierby tically feasible collaborated file GSTR-3B only on a New Delhi/Bengaluru, ment to go ahead with the the summer of 2021, its new 30.5 for distribution with quarterly basis kicks in, December 7 Human trials in both urban & AstraZeneca ground-breaking ceremony ownersannounced. -

SOUVENIR DESIGNS.Cdr
SOUVENIR A Glimpse into HEAL Born with the objective of facilitating meaningful communication between journalists, health communicators, research scientists, doctors and policy makers, we started as Health Essayists and Authors League to conceptualise and implement comprehensive education and intervention based health awareness campaigns in India. HEAL Foundation has sought and availed support of health journalists and writers from across the country to achieve its mission of a healthy and aware India. Health Writers and Editors Conventions and Health Writers Workshops are some of our initiatives to empower and strengthen health journalists and give them a sense of purpose. The Foundation works ceaselessly to create various knowledge disseminating opportunities for the media fraternity on a host of health issues impacting the Indian society. HEAL is committed to the cause of improving, innovating and to implementing healthcare delivery in the country so as to help realize the goal of achieving a high standard of health awareness in the society. HEAL founded The National Health Writers & Editors Convention as a platform for spreading awareness around pressing health issues of the country. The conventions have provided the ideal opportunity for health writers to comprehend and understand new developments in the field of healthcare. We are always open for partnership and association with like minded individuals and organisations to promote health awareness and improve health seeking behaviour of the Indian population. To know more or connect with us logon to www.healfoundaon.in 2 Message from the Founder In the last 14 years, we have played catalysts to minimum 10,000 health related stories covering almost 100% of the Indian health media. -

Towards a Healthier India
ISSUE 01 VOLUME 05 VOLUME JULY-AUGUST 2018 TOWARDS A HEALTHIER INDIA Focus on accessibility, affordability, and quality STATE FOCUS: Punjab Exclusive interview with FORCE FOR GOOD: CMCA INDIA MADE: TTK Prestige Dr Vinod K Paul, Member, NITI Aayog 1 CEO SPEAK India Now Business and Economy DEAR READERS, India’s promise is driven by fundamental growth drivers including a favourable demographic profile, a skilled workforce, an emerging middle class, a strong entrepreneurial culture, rising productivity, and a resilient private sector. The country’s strong economic fundamentals, high rates of GDP growth, and long-term potential make it an attractive destination for business and investment across borders. With a growth rate of 7.7% in the fourth quarter of 2017-18 (Central Statistics Office), the Indian economy regained the tag of being the fastest growing economy globally. Given the high levels of global interest in India and to promote a better understanding of the present progress and long-term potential of the country’s economy, IBEF launched an exclusive India Study in April 2018 titled India: Pushing the Right Levers. It highlights some of the major game changers expected to transform the Indian economy in the coming decade. The themes cover a host of areas including developments in the agri-value chain, measures to boost manufacturing, smart urbanisation, increasing renewable energy capacity, evolving a startup ecosystem, e-governance, new phase of consumerism, and GST. The study is accessible on the link https://www.ibef.org/research/india-study. IBEF will be continuously tracking these themes and providing updates and new insights for the benefit of its online audiences. -

Artificial Intelligence for Healthcare Insights from India Contents
Research Paper Claire Muñoz Parry and Urvashi Aneja Centre for Universal Health & Asia-Pacific Programme | July 2020 Artificial Intelligence for Healthcare Insights from India Contents Summary 2 1 Introduction 3 2 AI and Healthcare 4 3 AI in Healthcare in India: Applications, Challenges and Risks 11 4 Conclusion: Considerations for AI and Healthcare in India 23 References 24 About the Authors 31 Acknowledgments 32 1 | Chatham House Artificial Intelligence for Healthcare: Insights from India Summary • AI, the use of coded computer software routines with specific instructions to perform tasks for which a human brain is considered necessary, is providing the healthcare sector with new advances that are being hailed as game changers. • The risk and challenges of integrating AI into healthcare are closely related to the use of the data needed to feed AI systems. Issues around quality, safety, governance, privacy, consent and ownership must all be properly addressed. A lack of explainability, as it is almost impossible to understand how AI arrived at a specific decision, also points to a potential lack of trust in AI systems. • India provides a case study of how a country is actively promoting the use of AI to address healthcare needs. However, the deployment of AI in India is still at a very nascent stage, particularly for clinical interventions. • The challenge of delivering quality healthcare at scale presents a strong case for developing AI-based solutions for healthcare in India. However, a complex health landscape involving numerous stakeholders, competing priorities, entrenched incentive systems and institutional cultures give rise to a range of challenges and risks across the stages of development, adoption and deployment. -
Healthcare Evolution Curative to Preventive
Healthcare Evolution Curative to Preventive APRIL Valuation | Investment Banking | Restructuring Transaction Services | Transaction Tax 2021 1 Healthcare Ecosystem Page No. 04 Global Healthcare 2 Industry Page No. 06 Indian Healthcare 3 Industry Page No. 09 4 Hospitals Page No. 16 5 Telemedicine Page No. 25 6 Diagnostics Page No. 33 Healthcare Industry - 7 Way Forward Page No. 41 Foreword ‘Unprecedented’ is a word we have all heard so many times in the past few months. The contours and contents of the healthcare ecosystem are rapidly evolving across the world due to major disruption caused by COVID-19 pandemic. In this current situation, where the fear of COVID-19 has spread across the world and countries are preparing to combat the second wave, technology is helping us remain united and Healthcare industry is no exception. While the delivery of healthcare varies significantly across developing and developed countries and even within them, there is a common objective to minimize disease burden and maximize the reach of quality care. While providing healthcare is widely recognised as the responsibility of government, private capital and expertise are increasingly viewed as welcome sources to induce efficiency and innovation. Indian Healthcare is complex due to the multi-layered architecture of health system administration. These include whether it gets public (Central or state government) or private funding, what location it covers (rural or urban), and what demography and prevalent diseases it covers. The big question around Indian Healthcare has always been around accessibility and affordability for most of the population. On one hand, it is largely financed by Out-of-pocket payments (comprising of more than 60% through private savings) and remains unaffordable for a large part of population. -
Momentous Rise of the Indian Start-Up Ecosystem Edition 2015
Start-up India – Momentous Rise of the Indian Start-up Ecosystem Edition 2015 Supported by Google for Entrepreneurs Start-up India – Momentous Rise of the Indian Start-up Ecosystem Edition 2015 Supported by Google for Entrepreneurs Copyright ©2015 International Youth Centre, Teen Murti Marg, Chanakyapuri New Delhi - 110 021, India Phone: 91-11-23010199, Fax: 91-11-23015452 Email: [email protected] Published by NASSCOM Designed & Produced by MKM CREATIVE Email : [email protected] Disclaimer The information contained herein has been obtained from sources believed to be reliable. NASSCOM disclaims all warranties as to the accuracy, completeness or adequacy of such information. NASSCOM shall have no liability for errors, omissions or inadequacies in the information contained herein, or for interpretations thereof. The material in this publication is copyrighted. No part of this report can be reproduced either on paper or electronic media without permission in writing from NASSCOM. request for permission to reproduce any part of the report may be sent to NASSCOM. Usage of Information Forwarding/copy/using in publications without approval from NASSCOM will be considered as infringement of intellectual property rights. This report is solely for the use of NASSCOM. No Part of it may be quoted, circulated or reproduced for distribution outside the client organization without prior written approval from NASSCOM and Zinnov Foreword India has truly become a start-up ‘Nation’. Since 2010, power, healthcare, employment, and education. The Indian technology start-ups landscape has seen a Further, start-ups are empowering SMBs by tremendous growth towards creation of innovative start- enhancing their customer reach and overall ups and India, a home to a new breed of young start- business productivity. -

ADINET E-News Digest No. 24 September 2016
ADINET e-News Digest No. 24 September 2016 Contents Page no. Coming Next Feb: `World's largest' student tracker @ Shala 2 Asmita Yojna (SAY), a ready database of 25 crore school- going children Elsevier denies it will force SSRN users on to other services 2 Mumbai police diary: In love with books — the other side of 2 men in khaki Online Courses to count towards college degree, but only 3 up to 20% Inform Others! – Career in Knowledge Management 3 Rare Indian Recordings From 1900s Made Available by 4 British Library India is finally on the path of a truly digital nation 5 ‘With thousands of manuscripts left to be rewritten, it is a 5 race against time’ Now, talk to a doctor online 6 Wearable keyboard lets users Tap to type 7 Study Shows Link Between School Librarians and Higher 7 Test Scores Should All Research Papers Be Free? 7 Web 4.0 is coming – electronic agents will have an answer 8 to everything The Wonderful World of Wiki 9 Food for Thought 10 A D I N E T e - N e w s D i g e s t N o . 2 4 S e p t e m b e r 2 0 1 6 Page 1 2 2 2 0 1 6 2 0 1 5 Coming Next Feb: `World's Purchase of research repository largest' student tracker @ has horrified open access Shala Asmita Yojna (SAY), a advocates who fear acquisition ready database of 25 crore marks attempt to maintain school-going children control over publishing Come February 2017 and the The publishing giant Elsevier has government will launch probably insisted that it will not force the world's largest student- academics to sign up to its other tracking programme, Shala services in order to use the newly Asmita Yojna (SAY), that will acquired Social Science Research have a ready database of 25 Network (SSRN) repository.