CTRI Trial Data
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

15 Sub Ptt MSB-TBM-CGL DOWN WEEK DAYS
CHENNAI BEACH - TAMBARAM - CHENGALPATTU DOWN WEEK DAYS Train Nos 40501 40001 40503 40505 40507 40701 40509 Kms Stations CJ 0 Chennai Beach d 03:55 04:15 04:35 04:55 05:15 05:30 05:50 2 Chennai Fort d 03:59 04:19 04:39 04:59 05:19 05:34 05:54 4 Chennai Park d 04:02 04:22 04:42 05:02 05:22 05:37 05:57 5 Chennai Egmore d 04:05 04:25 04:45 05:05 05:25 05:40 06:00 7 Chetpet d 04:08 04:28 04:48 05:08 05:28 05:43 06:03 9 Nungambakkam d 04:11 04:31 04:51 05:11 05:31 05:46 06:06 10 Kodambakkam d 04:13 04:33 04:53 05:13 05:33 05:48 06:08 12 Mambalam d 04:15 04:35 04:55 05:15 05:35 05:50 06:10 13 Saidapet d 04:18 04:38 04:58 05:18 05:38 05:53 06:13 16 Guindy d 04:21 04:41 05:01 05:21 05:41 05:56 06:16 18 St.Thomas Mount d 04:24 04:44 05:04 05:24 05:44 05:59 06:19 19 Palavanthangal d 04:27 04:47 05:07 05:27 05:47 06:02 06:22 21 Minambakkam d 04:30 04:50 05:10 05:30 05:50 06:05 06:25 22 Tirusulam d 04:32 04:52 05:12 05:32 05:52 06:07 06:27 24 Pallavaram d 04:35 04:55 05:15 05:35 05:55 06:10 06:30 26 Chrompet d 04:38 04:58 05:18 05:38 05:58 06:13 06:33 29 Tambaram Sanatorium d 04:41 05:01 05:21 05:41 06:01 06:16 06:36 30 Tambarm a 05:10 d 04:50 05:30 05:50 06:10 06:25 06:45 34 Perungulathur d 04:56 05:36 05:56 06:16 06:32 06:56 36 Vandalur d 04:59 05:39 05:59 06:19 06:35 06:59 39 Urappakkam d 05:03 05:43 06:03 06:23 06:39 07:03 42 Guduvancheri d 05:07 05:47 06:07 06:27 06:43 07:07 44 Potheri d 05:11 05:51 06:11 06:31 06:47 07:11 46 Kattangulathur d 05:14 05:54 06:14 06:34 06:50 07:14 47 Maraimalai Nagar d 05:16 05:56 06:16 06:36 06:52 07:16 51 Singaperumal -

The Chennai Comprehensive Transportation Study (CCTS)
ACKNOWLEDGEMENT The consultants are grateful to Tmt. Susan Mathew, I.A.S., Addl. Chief Secretary to Govt. & Vice-Chairperson, CMDA and Thiru Dayanand Kataria, I.A.S., Member - Secretary, CMDA for the valuable support and encouragement extended to the Study. Our thanks are also due to the former Vice-Chairman, Thiru T.R. Srinivasan, I.A.S., (Retd.) and former Member-Secretary Thiru Md. Nasimuddin, I.A.S. for having given an opportunity to undertake the Chennai Comprehensive Transportation Study. The consultants also thank Thiru.Vikram Kapur, I.A.S. for the guidance and encouragement given in taking the Study forward. We place our record of sincere gratitude to the Project Management Unit of TNUDP-III in CMDA, comprising Thiru K. Kumar, Chief Planner, Thiru M. Sivashanmugam, Senior Planner, & Tmt. R. Meena, Assistant Planner for their unstinted and valuable contribution throughout the assignment. We thank Thiru C. Palanivelu, Member-Chief Planner for the guidance and support extended. The comments and suggestions of the World Bank on the stage reports are duly acknowledged. The consultants are thankful to the Steering Committee comprising the Secretaries to Govt., and Heads of Departments concerned with urban transport, chaired by Vice- Chairperson, CMDA and the Technical Committee chaired by the Chief Planner, CMDA and represented by Department of Highways, Southern Railways, Metropolitan Transport Corporation, Chennai Municipal Corporation, Chennai Port Trust, Chennai Traffic Police, Chennai Sub-urban Police, Commissionerate of Municipal Administration, IIT-Madras and the representatives of NGOs. The consultants place on record the support and cooperation extended by the officers and staff of CMDA and various project implementing organizations and the residents of Chennai, without whom the study would not have been successful. -
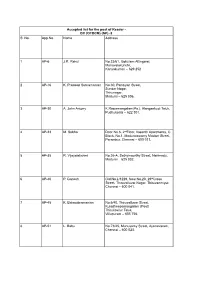
S. No. App.No. Name Address 1 AP-6 J.R. Rahul 2 AP-16 K. Pradeep
Accepted list for the post of Reader - BC (OTBCM) (NP) -2 S. No. App.No. Name Address 1 AP-6 J.R. Rahul No.23/61, Gokulam Attingarai, Manavalakurichi, Kanyakumari – 629 252 2 AP-16 K. Pradeep Subramanian No.30, Pandiyan Street, Sundar Nagar, Thirunagar, Madurai – 625 006. 3 AP-30 A. John Antony K.Rasiamangalam(Po.), Alangankudi Taluk, Pudhukottai – 622 301. 4 AP-33 M. Subha Door No.6, 2ndFloor, Vasanth Apartments, C Block, No.1, Maduraiswamy Madam Street, Perambur, Chennai – 600 011. 5 AP-35 R. Vijayalakshmi No.26-A, Sathymoorthy Street, Narimedu, Madurai – 625 002. 6 AP-40 P. Ganesh Old No.L/1229, New No.20, 29thCross Street, Thiruvalluvar Nagar, Thiruvanmiyur, Chennai – 600 041. 7 AP-45 K. Balasubramanian No.6/40, Thiruvalluvar Street, Kuladheepamangalam (Post) Thirukovilur Taluk, Villupuram – 605 756. 8 AP-51 L. Babu No.73/45, Munusamy Street, Ayanavaram, Chennai – 600 023. 9 AP-56 S. Barkavi No.168, Sivaji Nagar, Veerampattinam, Pondicherry – 605 007. 10 AP-62 R.D. Mathanram No.57, Jeeyar Narayanapalayam St, Kanchipuram – 631 501 11 AP-77 M.Parameswari No.8/4, Alagiri Nagar, 1ststreet, Vadapalani, chennai -26. 12 AP-83 G. Selva Kumari No. 12, G Block, Singara thottam, Police Quarters, Old Washermen pet, Chennai 600 021 13 AP-89 P. Mythili No.137/64, Sanjeeviroyan Koil Street, Old Washermenpet, Chennai – 600 021. 14 AP-124 K. Balaji No.11, Muthumariamman Koil Street, Bharath Nagar, Selaiyur, Chennai – 600 073. 15 AP-134 S. Anitha No.5/55-A, Main Road, Siruvangunam, Iraniyasithi Post, Seiyur Taluk, Kancheepuram – 603 312. -
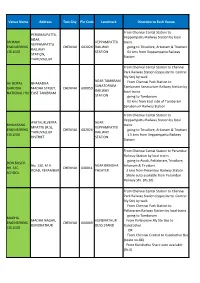
Venue Name Address Test City Pin Code Landmark Direction to Each Venue
Venue Name Address Test City Pin Code Landmark Direction to Each Venue From Chennai Cental Station to PERUMALPATTU, Veppampattu Railway Station by local NEAR SRI RAM VEPPAMPATTU trains VEPPAMPATTU ENGINEERING CHENNAI 602024 RAILWAY going to Trivallore, Arkonam & Tiruttani. RAILWAY COLLEGE STATION 01 kms from Veppampattu Railway STATION, Station THIRUVALLUR From Chennai Cental Station to Chennai Park Railway Station (opposite to Central Rly Stn) by walk NEAR TAMBRAM JAI GOPAL BHARADHA From Chennai Park Station to SANATORIUM GARODIA MADHA STREET, CHENNAI 600059 Tambaram Sanatorium Railway Station by RAILWAY NATIONAL HSS EAST TAMBRAM local trains STATION going to Tambaram 01 kms from East side of Tambaram Sanatorium Railway Station From Chennai Cental Station to Veppampattu Railway Station by local AYATHUR,VEPPA NEAR BHAJARANG trains MPATTU (R.S), VEPPAMPATTU ENGINEERING CHENNAI 602024 going to Trivallore, Arkonam & Tiruttani. THIRUVALLUR RAILWAY COLLEGE 1.5 kms from Veppampattu Railway DISTRICT STATION Station From Chennai Cental Station to Perambur Railway Station by local trains going to Avadi, Pattabiram,Trivallore, DON BOSCO No. 130, M.H. NEAR BRINDHA Arkonam & Tiruttani. HR. SEC. CHENNAI 600011 ROAD, PERAMBUR THEATER 2 kms from Perambur Railway Station SCHOOL Share auto available from Perambur Railway Stn. (Rs.10) From Chennai Cental Station to Chennai Park Railway Station (opposite to Central Rly Stn) by walk From Chennai Park Station to Pallavaram Railway Station by local trains going to Tambaram MADHA MADHA NAGAR, KUNDRATHUR From Pallavaram Rly Stn Bus to ENGINEERING CHENNAI 600069 KUNDRATHUR BUSS STAND Kundrathur. COLLEGE OR From Chennai Central to Kundrathur Bus (route no.88) From Kundrathu Share auto available (Rs.5) Dr. -

Tambaram - Chennai Beach up Sunday Tambaram - Chennai Beach up Sunday
Tambaram - Chennai Beach Up Sunday Tambaram - Chennai Beach Up Sunday Train Nos. 40302 40304 40306 40602 40308 40604 40606 40310 40608 40610 40312 40314 40752 40316 40612 Kms Stations 0 Tambaram d 4:00 4:20 4:40 4:55 5:15 5:30 5:45 6:00 6:10 6:30 6:45 7:00 7:15 7:30 7:45 2 Tambaram Sanatorium d 4:04 4:24 4:44 4:59 5:19 5:34 5:49 6:04 6:14 6:34 6:49 7:04 7:19 7:34 7:49 4 Chromepet d 4:07 4:27 4:47 5:02 5:22 5:37 5:52 6:07 6:17 6:37 6:52 7:07 7:22 7:37 7:52 6 Pallavaram d 4:10 4:30 4:50 5:05 5:25 5:40 5:55 6:10 6:20 6:40 6:55 7:10 7:25 7:40 7:55 8 Thirusulam d 4:13 4:33 4:53 5:08 5:28 5:43 5:58 6:13 6:23 6:43 6:58 7:13 7:28 7:43 7:58 10 Minambakkam d 4:15 4:35 4:55 5:10 5:30 5:45 6:00 6:15 6:25 6:45 7:00 7:15 7:30 7:45 8:00 12 Palavanthangal d 4:18 4:38 4:58 5:13 5:33 5:48 6:03 6:18 6:28 6:48 7:03 7:18 7:33 7:48 8:03 13 St.Thomas Mount d 4:21 4:41 5:01 5:16 5:36 5:51 6:06 6:21 6:31 6:51 7:06 7:21 7:36 7:51 8:06 15 Guindy d 4:24 4:44 5:04 5:19 5:39 5:54 6:09 6:24 6:34 6:54 7:09 7:24 7:39 7:54 8:09 17 Saidapet d 4:27 4:47 5:07 5:22 5:42 5:57 6:12 6:27 6:37 6:57 7:12 7:27 7:42 7:57 8:12 18 Mambalam d 4:30 4:50 5:10 5:25 5:45 6:00 6:15 6:30 6:40 7:00 7:15 7:30 7:45 8:00 8:15 20 Kodambakkam d 4:32 4:52 5:12 5:27 5:47 6:02 6:17 6:32 6:42 7:02 7:17 7:32 7:47 8:02 8:17 21 Nungambakkam d 4:34 4:54 5:14 5:29 5:49 6:04 6:19 6:34 6:44 7:04 7:19 7:34 7:49 8:04 8:19 23 Chetpet d 4:37 4:57 5:17 5:32 5:52 6:07 6:22 6:37 6:47 7:07 7:22 7:37 7:52 8:07 8:22 25 Chennai Egmore d 4:40 5:00 5:20 5:35 5:55 6:10 6:25 6:40 6:50 7:10 7:25 7:40 7:55 8:10 8:25 27 Chennai Park d 4:43 5:03 5:23 5:38 5:58 6:13 6:28 6:43 6:53 7:13 7:28 7:43 7:58 8:13 8:28 28 Chennai Fort d 4:46 5:06 5:26 5:41 6:01 6:16 6:31 6:46 6:56 7:16 7:31 7:46 8:01 8:16 8:31 30 Chennai Beach d 4:55 5:15 5:35 5:50 6:10 6:25 6:40 6:55 7:05 7:25 7:40 7:55 8:10 8:25 8:40 Tambaram - Chennai Beach Up Sunday Tambaram - Chennai Beach Up Sunday Train Nos. -

Auction-Retail-Shanoj-C-P-2021.Pdf
Retail Lending and Payment Group (South Zonal Office/Branch):Axis Bank-RAC, Arcot Plaza, Old No.38, New No.165, Arcot Road, Kodambakkam, Chennai - 600024 Axis Bank Ltd., 3rd Floor, Gigaplex, NPC – 1, TTC Industrial Area, Mugalsan Road, Airoli, Navi Mumbai – 400 708. Registered Office: “Trishul”, 3rd Floor Opp. Samartheshwar Temple Law Garden, Ellisbridge Ahmedabad – 380006 Public Notice for Sale/Auction of immovable properties {under SARFAESI Act read with proviso to Rule 8 (6) of the Security Interest (Enforcement) Rules} Whereas the Authorized Officer of Axis Bank Ltd. (hereinafter referred to as ‘the Bank’), under Securitisation and Reconstruction of Financial Assets and Enforcement of Security Interest Act, 2002 (in short ‘SARFAESI Act) and in exercise of powers conferred under Section 13(12) read with the Security Interest (Enforcement) Rules, 2002 issued Demand Notice under Sec. 13(2) of SARFAESI Act calling upon the below-mentioned Borrowers/Co-borrowers/mortgagors/Guarantors to repay the amount mentioned in the notice being the amount due together with further interest thereon at the contractual rate plus all costs charges and incidental expenses etc. till the date of payment within 60 days from the date of the said notice. The Borrowers/Co-borrowers/Mortgagors/Guarantors having failed to repay the above said amount within the specified period, the authorized officer has taken over physical possession in exercise of powers conferred under Section 13(4) of SARFAESI Act read with Security Interest (Enforcement) Rules, 2002, which -

Tamil Nadu Government Gazette
© [Regd. No. TN/CCN/467/2009-11. GOVERNMENT OF TAMIL NADU [R. Dis. No. 197/2009. 2010 [Price: Rs. 23.20 Paise. TAMIL NADU GOVERNMENT GAZETTE PUBLISHED BY AUTHORITY No. 27] CHENNAI, WEDNESDAY, JULY 14, 2010 Aani 30, Thiruvalluvar Aandu–2041 Part VI—Section 4 Advertisements by private individuals and private institutions CONTENTS PRIVATE ADVERTISEMENTS Pages Change of Names .. 1259-1316 Notice .. 1316 NOTICE NO LEGAL RESPONSIBILITY IS ACCEPTED FOR THE PUBLICATION OF ADVERTISEMENTS REGARDING CHANGE OF NAME IN THE TAMIL NADU GOVERNMENT GAZETTE. PERSONS NOTIFYING THE CHANGES WILL REMAIN SOLELY RESPONSIBLE FOR THE LEGAL CONSEQUENCES AND ALSO FOR ANY OTHER MISREPRESENTATION, ETC. (By Order) Director of Stationery and Printing. CHANGE OF NAMES My son, P. Manoj, born on 8th October 1996 (native My daughter, R. Harini, daughter of Thiru A.S. Ranganathan, district: Erode), residing at Old No. 2/26, New No. 1/79, born on 15th December 1993 (native district: Thiruvannamalai), Kongampalayam, Chittode, Erode-638 102, shall hencefroth residing at No. 122, Bharathi Street, V.G.P. Shanthi Nagar, be known as P. METHUNRAJ. Narayanapuram, Chennai-600 100, shall henceforth be known as R. SRIHARINI. K.R.E. PONGI. Chittode, 5th July 2010. (Father.) RAJALAKSHMI RANGAN. Chennai, 5th July 2010. (Mother.) My son, P. Yaswanth, born on 14th October 1999 (native I, M.C. Deepa, wife of Thiru Bhuvanesh Srinivasan, born district: Erode), residing at Old No. 2/26, New No. 1/79, on 21st May 1977 (native district: Kancheepuram), residing Kongampalayam, Chittode, Erode-638 102, shall henceforth at Old No. 85, New No. 34, Gengu Reddy Street, be known as K.E.P. -

566B Bus Time Schedule & Line Route
566B bus time schedule & line map 566B Iyyappanthangal View In Website Mode The 566B bus line (Iyyappanthangal) has 4 routes. For regular weekdays, their operation hours are: (1) Iyyappanthangal: 6:55 PM - 7:55 PM (2) Kovalam: 6:08 AM - 4:10 PM (3) Pattur: 7:30 AM - 2:10 PM (4) Tambaram: 8:25 AM - 6:25 PM Use the Moovit App to ƒnd the closest 566B bus station near you and ƒnd out when is the next 566B bus arriving. Direction: Iyyappanthangal 566B bus Time Schedule 32 stops Iyyappanthangal Route Timetable: VIEW LINE SCHEDULE Sunday 6:55 PM - 7:55 PM Monday 6:55 PM - 7:55 PM Tambaram Tuesday 6:55 PM - 7:55 PM Kadaperi Wednesday 6:55 PM - 7:55 PM T.B. Sanatorium Thursday 6:55 PM - 7:55 PM Tambaram Sanatorium Friday 6:55 PM - 7:55 PM Chromepet M.I.T. Flyover Saturday 6:55 PM - 7:55 PM Chromepet Saravana Store/Esi Hospital 566B bus Info Ponds Direction: Iyyappanthangal Stops: 32 Trip Duration: 48 min Palavaram Line Summary: Tambaram, Kadaperi, T.B. Sanatorium, Tambaram Sanatorium, Chromepet Tirususlam M.I.T. Flyover, Chromepet, Saravana Store/Esi Hospital, Ponds, Palavaram, Tirususlam, Tirusulam, Tirusulam Thirusoolam National Airport, Meenambakkam, Junction Of Palavanthangal & GST, Alandur Cement Thirusoolam National Airport Road, Alandur Depot, St.Thomas Mount, Cantonment Board (St. Thomas Mount Head Post Meenambakkam O∆ce), Kathipara Bridge Junction, Butt Road, Military Quarters, Contonment Cemetery, Chennai Junction Of Palavanthangal & GST Trade Centre, Ramapuram, Dlf And L&T, Moon Light, Mugalivakkam, Porur Sakthi Nagar, Porur, Retteri, Alandur Cement Road Ramachandra Medical College, Iyyappanthangal Depot Alandur Depot St.Thomas Mount Cantonment Board (St. -

CTRI Trial Data
PDF of Trial CTRI Website URL - http://ctri.nic.in Clinical Trial Details (PDF Generation Date :- Sun, 03 Oct 2021 08:02:30 GMT) CTRI Number CTRI/2020/06/025761 [Registered on: 09/06/2020] - Trial Registered Prospectively Last Modified On 22/04/2021 Post Graduate Thesis No Type of Trial Observational Type of Study Cross Sectional Study Study Design Non-randomized, Multiple Arm Trial Public Title of Study Population based cross sectional study for COVID 19 prophylaxis with Polyherbal Siddha formulation Kabasura Kudineer / Nilavembu kudineer in containment zones and non containment zones during 2020 pandemic in Tamil Nadu, South India Scientific Title of Population based cross sectional study for COVID 19 prophylaxis with Polyherbal Siddha Study formulation Kabasura Kudineer / Nilavembu kudineer in containment zones and non containment zones during 2020 pandemic in Tamil Nadu, South India Secondary IDs if Any Secondary ID Identifier NIL NIL Details of Principal Details of Principal Investigator Investigator or overall Name Dr R Meenakumari Trial Coordinator (multi-center study) Designation Director Affiliation National institute of Siddha Address National institute of siddha tambaram sanatorium National institute of siddha tambaram sanatorium Kancheepuram TAMIL NADU 600047 India Phone 9176534465 Fax Email [email protected] Details Contact Details Contact Person (Scientific Query) Person (Scientific Name Dr G J Christian Query) Designation Head of the Department , department of Noi Naadal Affiliation National institute of Siddha -

2 Bedroom Apartment / Flat for Sale in Pallavaram, Chennai (P17418751
https://www.propertywala.com/P17418751 Home » Chennai Properties » Residential properties for sale in Chennai » Apartments / Flats for sale in Pallavaram, Chennai » Property P17418751 2 Bedroom Apartment / Flat for sale in Pallavaram, Chennai 76 lakhs Radiance The Pride Luxury Apartments In Advertiser Details Chennai Pallavaram - Radiance The Pride Near Airport 77,Pammal Main Road,, Pallavaram, Chennai - 600078 (T… Area: 1068 SqFeet ▾ Bedrooms: Two Bathrooms: Two Floor: Second Total Floors: Five Facing: East Furnished: Unfurnished Transaction: New Property Price: 7,600,000 Rate: 7,116 per SqFeet Scan QR code to get the contact info on your mobile View all properties by OneRoof Properties Age Of Construction: Under Construction Possession: Within 3 Years Pictures Description Radiance The Pride luxury apartments in Chennai are located at Pallavaram, near airport. Radiance The Pride luxury apartments in Pallavaram is coming up on a sprawling 5.57 acres, with over 30 amenities to feed your senses. Well-connected and surrounded by life’s best, these homes are designed to please. Incredibly close to the airport, connected by buses and trains, close proximity to educational institutions, hospitals, offices, etc., this locality is perfect for families to flourish in. Come and experience Elevation Elevation The Pride of Pallavaram. *Tentative all inclusive price. PLC charges extra. Amenities: GYM Aerial View Drawing Room Swimming Pool Multipurpose Hall Yoga Deck CCTV Kitchen Bedroom Reading Lounge Mini Theatre Cricket Nets Kids Play Area Swimming -

MM Vol. XV No. 12.Pmd
Reg. No. TN/PMG (CCR) /814/04-05 Licence No. WPP 506/04-05 Registered with the Registrar of Newspapers for India under WE CARE FOR MADRAS THAT IS CHENNAI R.N. 53640/91 INSIDE The ration card nightmare Where Dhanammal lived MADRAS Aquarian display again Our timekeeper Harvard MUSINGS A batting metamorphosis Rs. 5 per copy Vol. XV No. 12 October 1-15, 2005 (Annual Subscription: Rs. 100/-) Madras AND Chennai Theyve both existed from the Citys beginnings (By S. Muthiah) Sir, I strongly suggest you order efore the British there was 1639 was by the Damarla broth- something while you wait for your car...! Bno Madras. There was no ers, the local Nayaks (gover- Chennai either. What there nors) of the Rajah of Chandra- Pick a car, any car were, were small villages like giri (representing the last ves- You know those melancholic Tiruvottriyur and Tiruvanm- tiges of the Vijayanagar King- hordes crowding hotel en- trances, gazing ineffectually in iyur and a fading town, Myla- dom of Kannada lineage but the direction where their loved pore, with its suburb Triplicane Telugu in speech). ones last disappeared? and, in between, scrub jungle, With the grant came a re- All part of the I-didnt-park-my- paddy fields and stretches of quest to name the new settle- car syndrome beach. I would be delighted to ment Chennapatnam, after Like any form of self-indulgence, be shown any parchment or their father, Chennappa, a valet parking also extracts a palm leaf manuscript or rock noble of the Chandragiri Court. price. -

Tambaram Municipality
Government of Tamil Nadu Tamil Nadu Urban Development Fund City Corporate cum Business Plan Tambaram Municipality FINAL REPORT October 2007 Wilbur Smith Associates Private Limited TN_CCP-BP_Tambaram Abbreviations and Acronyms BOT : Build, Operate and Transfer BPL : Below Poverty Line BT : Black Top CAA : Constitution Amendment Act CAGR : Compounded Annual Growth Rate CC : Cement Concrete CCP : City Corporate Plan CMA : Chennai Metropolitan Area CMDA : Chennai Metropolitan Development Authority CMWSSB : Chennai Metropolitan Water Supply and Sewerage Board CPHEEO : Central Public Health Environmental Engineering Organization CSC : Community Structure Component CUA : Chennai Urban Agglomeration DIC : District Industries Centre DPR : Detailed Project Report DWCUA : Development of Women and Children in Urban Areas EAR : Environmental Assessment Reports ECR : East Coast Road ELSR : Elevated Storage Reservoir ESF : Environmental and Social Framework ESR : Environmental and Social Report FOP : Financial and Operating Plan FY : Financial Year G.S.T. Road : Grand South Trunk Road gm : Grams GoI : Government of India GoTN : Government of Tamil Nadu gpcd : Grams per Capita per Day GLSR : Ground Level Storage Reservoir ISP : Integrated Sanitation Program IT : Information Technology Ha : Hectares HH : Households HSC : House Service Connection IPT : Intermediate Public Transport ISP : Integrated Sanitation Program kg : Kilograms LCS : Low Cost Sanitation Lit : Liters LL : Lakh Liters LPA : Local Planning Area lpcd : Liters Per Capita Per Day m : Metres