Scientific Meeting & Clinical Lab Expo
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

CONGRATULATIONS New World Team
MARCH 2021 CONGRATULATIONS New World Team • A S ABILASH & NEETHU ABILASH, KODAKARA • BEGUM AZIZA & MOHAMMED IBRAHIM, HYDERABAD • A S BHAGYASHREE , DAVANGERE • BEGUM HASEENA & ABDUL SATTAR, SHIMOGA • A UMERA BANU , DODDABALLAPUR • BEHERA CHINMAY , CUTTACK • AASHIMA , HANSI • BELERI NAGAPPA & B S PRATIMADEVI, KOPPAL • ABASAHEB GARJE KANTILAL & ANITA KANTILAL GARJE, • BHAGAT JOLLY , AGRA AHMED NAGAR • BHANAP SAMPADA SUHAS , AURANGABAD • ABRAHAM BENNY , KANNUR • BHANGE ROHINI DNYANDEO & RAVINDRA BANDOPANT • AFZAAL AHMED KHAN ABU HURAIRAH , AURANGABAD BODAKHE, NEVASA • AGADI MUKTHUM SAB & BABIJAN MUKTHUMSAB • BHARATH KUMAR AKULA & A SWATHI, AGAGI, UTTARA KANNADA MAHABUBNAGAR • AGARWAL SHEETAL , SUNDERGARH • BHARGAVA UDBHAV , NEW DELHI • AGRAWAL SONIKA , JASHPUR • BHARGAVI R & R DINESHKUMAR, COIMBATORE • ALAPATI DIVYA , WEST GODAVARI • BHAVANA , KARNAL • ALI KASEM & NASIMA BEGUM, SONITPUR • BHAVSAR SIDDHI DHAVAL , AHMEDABAD • AMBIKA HD & HD NAGABHUSHANA, ANANTHAPUR • BINESH , NANMANDA • AMIL , MEERUT • BISWAS SHARMISTHA , BARDHAMAN • ANASUYAMMA & VENKATARAMAPPA, ANANTHAPUR • BOBBILI DHANPAL REDDY & BOBBILI SWETHA, • ANIRUDDHA B V , CHICKMAGALUR HYDERABAD • ANJALI , KARNAL • BODRAVARA MUNIRAJA & BASAMMA P, DAVANGERE • ANJUM SHABANA & SARWAR NISHAT, DHANBAD • BROJEN SINGH THONGAM & THONGAM SUNIBALA, • ANNAPOORANI V & A PARUMAL, COIMBATORE THOUBAL • ANWARHUSSAIN KAKHANDKI MOHAMMED KHALID , • BUNKAR MOHAN LAL , RAJSAMAND BAGALKOT • BURADE CHAYA RAVINDRA & RAVINDRA SURYAKANT • ARCHANA SHASHI & SATISH KUMAR, PATNA BURADE, SOLAPUR • ARI KISHORE KUMAR -

Reg. No Name in Full Residential Address Gender Contact No
Reg. No Name in Full Residential Address Gender Contact No. Email id Remarks 20001 MUDKONDWAR SHRUTIKA HOSPITAL, TAHSIL Male 9420020369 [email protected] RENEWAL UP TO 26/04/2018 PRASHANT NAMDEORAO OFFICE ROAD, AT/P/TAL- GEORAI, 431127 BEED Maharashtra 20002 RADHIKA BABURAJ FLAT NO.10-E, ABAD MAINE Female 9886745848 / [email protected] RENEWAL UP TO 26/04/2018 PLAZA OPP.CMFRI, MARINE 8281300696 DRIVE, KOCHI, KERALA 682018 Kerela 20003 KULKARNI VAISHALI HARISH CHANDRA RESEARCH Female 0532 2274022 / [email protected] RENEWAL UP TO 26/04/2018 MADHUKAR INSTITUTE, CHHATNAG ROAD, 8874709114 JHUSI, ALLAHABAD 211019 ALLAHABAD Uttar Pradesh 20004 BICHU VAISHALI 6, KOLABA HOUSE, BPT OFFICENT Female 022 22182011 / NOT RENEW SHRIRANG QUARTERS, DUMYANE RD., 9819791683 COLABA 400005 MUMBAI Maharashtra 20005 DOSHI DOLLY MAHENDRA 7-A, PUTLIBAI BHAVAN, ZAVER Female 9892399719 [email protected] RENEWAL UP TO 26/04/2018 ROAD, MULUND (W) 400080 MUMBAI Maharashtra 20006 PRABHU SAYALI GAJANAN F1,CHINTAMANI PLAZA, KUDAL Female 02362 223223 / [email protected] RENEWAL UP TO 26/04/2018 OPP POLICE STATION,MAIN ROAD 9422434365 KUDAL 416520 SINDHUDURG Maharashtra 20007 RUKADIKAR WAHEEDA 385/B, ALISHAN BUILDING, Female 9890346988 DR.NAUSHAD.INAMDAR@GMA RENEWAL UP TO 26/04/2018 BABASAHEB MHAISAL VES, PANCHIL NAGAR, IL.COM MEHDHE PLOT- 13, MIRAJ 416410 SANGLI Maharashtra 20008 GHORPADE TEJAL A-7 / A-8, SHIVSHAKTI APT., Male 02312650525 / NOT RENEW CHANDRAHAS GIANT HOUSE, SARLAKSHAN 9226377667 PARK KOLHAPUR Maharashtra 20009 JAIN MAMTA -
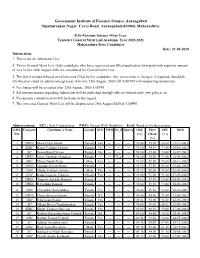
Government Institute of Forensic Science, Aurangabad Nipatniranjan Nagar, Caves Road, Aurangabad-431004, Maharashtra
Government Institute of Forensic Science, Aurangabad Nipatniranjan Nagar, Caves Road, Aurangabad-431004, Maharashtra. B.Sc-Forensic Science -First Year Tentative General Merit List-Academic Year 2020-2021 Maharashtra State Candidates Date: 21-08-2020 Instructions: 1. This is not an Admission List. 2. This is General Merit List. Only candidates who have registered and filled application form paid with requisite amount of fees before 20th August 2020 are considered for General Merit List. 3. This list is prepared based on information filled by the candidates. Any corrections or changes, if required, should be informed on email id: [email protected] before 23rd August, 2020 till 5.00 PM with supporting documents. 4. No change will be accepted after 23rd August, 2020 5.00PM. 5. All announcements regarding Admission will be published thorugh official website only: ww.gifsa.ac.in 6. No separate communication will be made in this regard. 7. The corrected General Merit List will be displayed on 24th August,2020 at 5.00PM. Abbreviations: NCL: Non-Creamylayer PWD: Person With Disability Ex-S: Ward of Ex-Servicemen GML Category Candidate's Name Gender NCL PWD Ex_S Sports HSC Phy+ SSC DOB NO. (%) Chem (%) (%) 1 OPEN More Prapti Manik Female Yes --- --- --- 96.00 35.50 68.80 13-11-2003 2 SEBC Barge Vaishnavi Sanjay Female Yes --- --- --- 95.60 94.50 93.40 05/09/2002 3 SC Gawai Kajal Devrao Female Yes --- --- --- 94.80 63.00 94.00 16/07/2002 4 OPEN Surve Vaishnavi Sandeep Female --- --- Yes --- 94.40 94.50 91.00 14-05-2002 5 SBC Nimje Piyush Vijay -

Professor Julie Audet<B>
University of Toronto Faculty, Staff, and Student Awards and Honours Governing Council Meeting October 23, 2008 Faculty & Staff Awards Professor Emeritus Paul Aird of forestry is the winner of the Endangered Species Stewardship Award, presented to him by Donna Cansfield, Ontario minister of natural resources, at a natural History Day event, held May 24 at Bonnechere Provincial Park in Renfrew County. Aird was honoured for his volunteer contributions related to Kirtland’s Warbler over the past 30-plus years, not only raising public awareness for this particular species at risk but for having made an important contribution towards recovery of this globally rare bird in Ontario. Keith Ambachtsheer, director of the Rotman International Centre for Pension Management, is the recipient of the James R. Vertin Award, given by the CFA (chartered financial analyst) Institute to recognize individuals who have produced a body of research notable for its relevance and enduring value to investment professionals. Ambachtsheer received the award July 22 at the 50th Financial Analysts Seminar, a CFA Institute conference hosted by the CFA Society of Chicago. Francesca Andrade of financial services is this year’s winner of U of T Scarborough’s Patrick Phillips Staff Award for outstanding service and commitment by a campus staff member, while Svetlana Mikhaylichenko of physical and environmental sciences is the recipient the D.R. Campbell Merit Award for enhancing the quality of life on campus. Professor Janet Potter of physical and environmental sciences is the winner of the Faculty Teaching Award. The Principal’s Awards were presented in June at an event hosted by Principal Franco Vaccarino. -

1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27
Case 2:16-cv-02373-SPL Document 1 Filed 07/15/16 Page 1 of 36 1 Mark D. Samson Ron Kilgard 2 KELLER ROHRBACK L.L.P. 3 3101 North Central Ave., Suite 1400 Phoenix, AZ 85012 4 Phone: (602) 248-0088, Fax (602) 248-2822 5 [email protected] [email protected] 6 Lynn Lincoln Sarko (pro hac vice application forthcoming) 7 Gretchen Freeman Cappio (pro hac vice application forthcoming) 8 T. David Copley 1201 Third Avenue, Suite 3200 9 KELLER ROHRBACK L.L.P. 10 Seattle, WA 98101-3052 (206) 623-1900, Fax (206) 623-3384 11 [email protected] [email protected] 12 [email protected] 13 Attorneys for Plaintiffs 14 15 UNITED STATES DISTRICT COURT DISTRICT OF ARIZONA 16 R.C., individually and on behalf of all others 17 similarly situated, No. 18 Plaintiff, 19 CLASS ACTION COMPLAINT v. 20 JURY TRIAL DEMANDED THERANOS, INC., a California 21 Corporation, and WALGREENS BOOTS 22 ALLIANCE, INC., a Delaware Corporation, 23 Defendants. 24 25 26 27 COMPLAINT 28 1 Case 2:16-cv-02373-SPL Document 1 Filed 07/15/16 Page 2 of 36 1 I. INTRODUCTION 2 1. Blood tests are a critical component of a patient’s healthcare. Patients, as well as 3 their doctors, rely on blood tests to detect conditions and use those results to help determine which 4 course of treatment is or isn’t needed. Inaccurate blood test results can contribute to serious 5 6 conditions going undetected, to treatable conditions growing worse unnoticed, to patients forgoing 7 medications they should take or taking medications they shouldn’t. -

DRUG DISCOVERY SUMMIT: Enabling Early Stage Drug Discovery and Venture Creation
PRESENT: DRUG DISCOVERY SUMMIT: Enabling Early Stage Drug Discovery and Venture Creation Event Program JLABS@Toronto Tuesday June 11, 2019 MaRS Centre, West Tower 1:00 – 6:00 PM 661 University Avenue, Suite 1300 Toronto, ON, Canada M5G 0B7 POWERED BY: WELCOME MaRS Innovation and our partner Evotec SE are pleased to welcome you to our inaugural LAB150 Drug Discovery Summit. We would like to thank Allan Miranda and the JLABS team for hosting us here today in this spectacular facility! It has been 18 months since we launched LAB150, a program designed to enable translation of academic discoveries into medicines from MaRS Innovation (MI) Members. With five projects approved and funded and one project graduated from the program and negotiating follow-on investment, we are off to an incredible start! The LAB150 program, paired with MI’s venture creation and investment model, provides a leading platform for life sciences venture creation and growth in Canada. Over the last decade, MI has created over 50 companies that have attracted and retained seasoned management, created over 400 STEM jobs and raised over $350M in direct third-party capital. With LAB150, we look forward to building on this success in the coming decade and beyond. Today you will hear from entrepreneurs and scientists regarding their experiences with the LAB150 program. In addition, we are pleased to be joined by scientific experts from Evotec who will share emerging trends in the drug discovery space. Last but not least, early stage investors will share their tips and tricks for securing capital for building therapeutic ventures in Canada. -

Theranos Phenomenon – Part 5: Theranos’ Presentation at the American Association for Clinical Chemistry Annual Conference 2016
Clin Chem Lab Med 2016; 54(10): e313–e314 Letter to the Editor Eleftherios P. Diamandis* and Mario Plebani Theranos phenomenon – Part 5: Theranos’ presentation at the American Association for Clinical Chemistry Annual Conference 2016 DOI 10.1515/cclm-2016-0737 Master, two highly recognized and respected clinical Accepted for publication August 18, 2016; previously published chemists, who did a good job in asking pertinent ques- online August 30, 2016 tions to Ms. Holmes and her associates. In this respect, AACC lived up to the expectations of high standards and Keywords: diagnostics; new technologies. impartiality for this presentation. Holmes presented to a large and curious, if not To the Editor, hostile, audience. She avoided talking about Theranos’ past and the difficulties of her company [1–7]. She also The widely anticipated presentation of Theranos at the made it clear that her presentation would focus on the Annual Conference of the American Association for future, not the past, and she distanced herself from the Clinical Chemistry (AACC) was finally given on Monday, previous “Edison” instrument and introduced a new August 1st, 2016 at the Philadelphia Convention Center. analyzer named “MiniLab”. In the first part of her pres- This journal has followed the Theranos story closely over entation, Ms. Holmes described the engineering behind the last 2 years and provided frequent updates [1–4]. Eliza- the MiniLab and explained that it is a compact desktop beth Holmes, the Chief Executive of Theranos, presented device that houses a mini spectrophotometer, a mini- to an audience of over 2500 clinical chemists and other luminometer, and mini-flow-cytometer and a mini-PCR laboratory scientists, as well as to an impressive number machine, along with a centrifuge. -

Name of the Centre : DAV Bachra No
Name of the Centre : DAV Bachra No. Of Students : 474 SL. No. Roll No. Name of the Student Father's Name Mother's Name 1 19002 AJAY ORAON NIRMAL ORAON PATI DEVI 2 19003 PUJA KUMARI BINOD PARSAD SAHU SUNITA DEVI 3 19004 JYOTSANA KUMARI BINOD KUMAR NAMITA DEVI 4 19005 GAZAL SRIVASTVA BABY SANTOSH KUMAR SRIVASTAVA ASHA SRIVASTVA 5 19006 ANURADHA KUMARI PAPPU KUMAR SAH SANGEETA DEVI 6 19007 SUPRIYA KUMARI DINESH PARSAD GEETA DEVI 7 19008 SUPRIYA KUMARI RANJIT SHINGH KIRAN DEVI 8 19009 JYOTI KUMARI SHANKAR DUBEY RIMA DEVI 9 19010 NIDHI KUMARI PREM KUMAR SAW BABY DEVI 10 19011 SIMA KUMARI PARMOD KUMAR GUPTA SANGEETA DEVI 11 19012 SHREYA SRIVASTAV PRADEEP SHRIVASTAV ANIMA DEVI 12 19013 PIYUSH KUMAR DHANANJAY MEHTA SANGEETA DEVI 13 19014 ANUJ KUMAR UPENDRA VISHWAKARMA SHIMLA DEVI 14 19015 MILAN KUMAR SATYENDRA PRASAD YADAV SHEELA DEVI 15 19016 ABHISHEK KUMAR GUPTA SANT KUMAR GUPTA RINA DEVI 16 19017 PANKAJ KUMAR MUKESH KUMAR SUNITA DEVI 17 19018 AMAN KUMAR LATE. HARISHAKAR SHARMA RINKI KUMARI 18 19019 ATUL RAJ RAJESH KUMAR SATYA RUPA DEVI 19 19020 HARSH KUMAR SHAILENDRA KR. TIWARY SNEHLATA DEVI 20 19021 MUNNA THAKUR HIRA THAKUR BAIJANTI DEVI 21 19022 MD.FARID ANSARI JARAD HUSSAIN ANSARI HUSNE ARA 22 19023 MD. JILANI ANSARI MD.TAUFIQUE ANSARI FARZANA BIBI 23 19024 RISHIKESH KUNAL DAMODAR CHODHARY KAMLA DEVI 24 19025 VISHAL KR. DUBEY RAJEEV KUMAR DUBEY KIRAN DEVI 25 19026 AYUSH RANJAN ANUJ KUMAR DWIVEDI ANJU DWIVEDI 26 19027 AMARTYA PANDEY SATISH KUMAR PANDEY REENA DEVI 27 19028 SHIWANI CHOUHAN JAYPAL SINGH MAMTA DEVI 28 19029 SANDEEP KUMAR MEHTA -

State District Branch Address Centre Ifsc Contact1 Contact2 Contact3 Micr Code
STATE DISTRICT BRANCH ADDRESS CENTRE IFSC CONTACT1 CONTACT2 CONTACT3 MICR_CODE ANDAMAN NO 26. MG ROAD AND ABERDEEN BAZAR , NICOBAR PORT BLAIR -744101 704412829 704412829 ISLAND ANDAMAN PORT BLAIR ,A & N ISLANDS PORT BLAIR IBKL0001498 8 7044128298 8 744259002 UPPER GROUND FLOOR, #6-5-83/1, ANIL ANIL NEW BUS STAND KUMAR KUMAR ANDHRA ROAD, BHUKTAPUR, 897889900 ANIL KUMAR 897889900 PRADESH ADILABAD ADILABAD ADILABAD 504001 ADILABAD IBKL0001090 1 8978899001 1 1ST FLOOR, 14- 309,SREERAM ENCLAVE,RAILWAY FEDDER ROADANANTAPURA ANDHRA NANTAPURANDHRA ANANTAPU 08554- PRADESH ANANTAPUR ANANTAPUR PRADESH R IBKL0000208 270244 D.NO.16-376,MARKET STREET,OPPOSITE CHURCH,DHARMAVA RAM- 091 ANDHRA 515671,ANANTAPUR DHARMAVA 949497979 PRADESH ANANTAPUR DHARMAVARAM DISTRICT RAM IBKL0001795 7 515259202 SRINIVASA SRINIVASA IDBI BANK LTD, 10- RAO RAO 43, BESIDE SURESH MYLAPALL SRINIVASA MYLAPALL MEDICALS, RAILWAY I - RAO I - ANDHRA STATION ROAD, +91967670 MYLAPALLI - +91967670 PRADESH ANANTAPUR GUNTAKAL GUNTAKAL - 515801 GUNTAKAL IBKL0001091 6655 +919676706655 6655 18-1-138, M.F.ROAD, AJACENT TO ING VYSYA BANK, HINDUPUR , ANANTAPUR DIST - 994973715 ANDHRA PIN:515 201 9/98497191 PRADESH ANANTAPUR HINDUPUR ANDHRA PRADESH HINDUPUR IBKL0001162 17 515259102 AGRICULTURE MARKET COMMITTEE, ANANTAPUR ROAD, TADIPATRI, 085582264 ANANTAPUR DIST 40 ANDHRA PIN : 515411 /903226789 PRADESH ANANTAPUR TADIPATRI ANDHRA PRADESH TADPATRI IBKL0001163 2 515259402 BUKARAYASUNDARA M MANDAL,NEAR HP GAS FILLING 91 ANDHRA STATION,ANANTHAP ANANTAPU 929710487 PRADESH ANANTAPUR VADIYAMPETA UR -

Annual Return – MGT-7
FORM NO. MGT-7 Annual Return [Pursuant to sub-Section(1) of section 92 of the Companies Act, 2013 and sub-rule (1) of rule 11of the Companies (Management and Administration) Rules, 2014] Form language English Hindi Refer the instruction kit for filing the form. I. REGISTRATION AND OTHER DETAILS (i) * Corporate Identification Number (CIN) of the company L14102TG1991PLC013299 Pre-fill Global Location Number (GLN) of the company * Permanent Account Number (PAN) of the company AABCP2100Q (ii) (a) Name of the company POKARNA LIMITED (b) Registered office address 1ST FLOOR, 105,SURYA TOWERS, SECUNDERABAD. A.P Telangana 500003 India (c) *e-mail ID of the company [email protected] (d) *Telephone number with STD code 04027897722 (e) Website www.pokarna.com (iii) Date of Incorporation 09/10/1991 (iv) Type of the Company Category of the Company Sub-category of the Company Public Company Company limited by shares Indian Non-Government company (v) Whether company is having share capital Yes No (vi) *Whether shares listed on recognized Stock Exchange(s) Yes No Page 1 of 15 (a) Details of stock exchanges where shares are listed S. No. Stock Exchange Name Code 1 B.S.E Limited 1 2 National Stock Exchange of India 1,024 (b) CIN of the Registrar and Transfer Agent U72400TG2017PTC117649 Pre-fill Name of the Registrar and Transfer Agent KFIN TECHNOLOGIES PRIVATE LIMITED Registered office address of the Registrar and Transfer Agents Selenium, Tower B, Plot No- 31 & 32, Financial District, Nanakramguda, Serilingampally (vii) *Financial year From date 01/04/2020 (DD/MM/YYYY) To date 31/03/2021 (DD/MM/YYYY) (viii) *Whether Annual general meeting (AGM) held Yes No (a) If yes, date of AGM (b) Due date of AGM 30/09/2021 (c) Whether any extension for AGM granted Yes No (f) Specify the reasons for not holding the same II. -
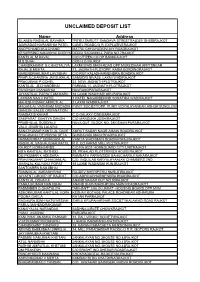
Unclaimed Deposit List
UNCLAIMED DEPOSIT LIST Name Address SILABEN RASIKLAL BAVARIA "PITRU SMRUTI" SANGAVA STREETRAJDEV SHERIRAJKOT JAMNADAS NARANBHAI PATEL CANEL ROADC/O R. EXPLASIVERAJKOT ANOPCHAND MULCHAND MATRU CHHAYADIGVIJAY ROADRAJKOT KESARISING NANJIBHAI DODIYA DODIA SADANMILL PARA NO.7RAJKOT KANTILAL M RAVAL C/O CITIZEN CO.OP.BANKRAJKOT M S SHAH CCB.H.O.RAJKOT CHANDRAKANT G CHHOTALIYA LAXMI WADI MAIN ROAD OPP MURLIDHAR APPTSNEAR RAJAL B MEHTA 13, JAGNATH PLOTOPP. KADIA BORDINGRAJKOT NARENDRAKUMAR LAVJIBHAI C/O RUP KALADHARMENDRA ROADRAJKOT PRAFULCHANDRA JAYSUKHLAL SAMUDRI NIVAS5, LAXMI WADIRAJKOT PRAGJIBHAI P GOHEL 22. NEW JAGNATH PLOTRAJKOT KANTILAL JECHANDBHAI PARIMAL11, JAGNATH PLOTRAJKOT RAYDHAN CHANABHAI NAVRANGPARARAJKOT JAYANTILAL PARSOTAM MARU 18 LAXMI WADIHARI KRUPARAJKOT LAXMAN NAGJI PATEL 1 PATEL NAGARBEHIND SORATHIA WADIRAJKOT MAHESHKUMAR AMRUTLAL 2 LAXMI WADIRAJKOT MAGANLAL VASHRAM MODASIA PUNIT SOCIETYOPP. PUNIT VIDYALAYANEAR ASHOK BUNGLOW GANESH SALES ORGANATION RAMDAS B KAHAR C.O GALAXY CINEMARAJKOT SAMPARAT SAHITYA SANGH C/O MANSUKH JOSHIRAJKOT PRABHULAL BUDDHAJI NAVA QUT. BLOCK NO. 28KISHAN PARARAJKOT VALJI JIVABHIA LALKIYA SANATKUMAR KANTILAL DAVE AMRUT SADAR NAGR AMAIN ROADRAJKOT BHAGWANJI DEVSIBHAI SETA GUNDAVADI MAIN ROADRAJKOT HASMUKHRAY CHHAGANLAL VANIYA WADI MAIN ROADNATRAJRAJKOT RASIKLAL VAGHAJIBHAI PATEL R.K. CO.KAPAD MILL PLOTRAJKOT RAJKOT HOMEGARDS C/O RAJKOT HOMEGUARD CITY UNITRAJKOT NITA KANTILAL RATHOD 29, PRHALAD PLOTTRIVEDI HOUSERAJKOT DILIPKUMAR K ADESARA RAMNATH PARAINSIDE BAHUCHARAJI NAKARAJKOT PRAVINKUMAR CHHAGANLAL -

Table of Contents
����������������������� �������� ������������������������� Table of Contents INTRODUCTION & OVERVIEW 2005-2006 4 HONOURS/AWARDS/APPOINTMENTS 6 Appointments 6 Awards 7 CLINICAL SERVICES 8 Patient Services 8 Clinical Biochemistry 8 Hematopathology & Blood Transfusion 9 Rapid Response Laboratory 9 Pathology 10 Laboratory Genetics 11 Andrology 12 LABORATORY INFORMATICS 13 Laboratory Information System 13 Expanded Interfaces 13 Citrix Support 13 Web Site/Communications 13 Paradigm Document Control System 14 Digital Imaging 14 RENOVATIONS & RELOCATIONS 15 QUALITY PROGRAMS 16 Accreditations, Regulatory Certifications/Registrations 16 External Quality Assessment Programs 17 Continuous Quality Improvement Activities: Indicators 18 Quality Management Team Activities: 2005-2006 18 EDUCATION 19 Educational Activities July 2005 – June 2006 19 Education Committee 19 Medical Education 19 Research Student Programs 24 Technical Education 25 SPONSORED RESEARCH AND INNOVATION 27 PRESENTATIONS & ABSTRACTS July 1, 2005 – June 30, 2006 32 SCIENTIFIC PAPERS July 1, 2005 – June 30, 2006 50 BOOKS & BOOK CHAPTERS 69 APPENDIX A - Medical and Scientific Staff as at June 30, 2006 71 APPENDIX B - Technical & Support Staff as at June 30, 2005 73 APPENDIX C - Technical & Support Staff Changes 75 APPENDIX D - Residents 76 APPENDIX E - Fellows 77 APPENDIX F - Graduate Students 2005-2006 78 APPENDIX G - Post Doctoral Students 81 APPENDIX H - Summer Students May – August 2005 82 APPENDIX I - Reportable Tests 83 APPENDIX J - External Service Linkages 85 APPENDIX K - April