CTRI Trial Data
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

UTTARAKHAND Sectorwise Gross District Domestic Product (GDDP ) for the Year : 2005-06 ( at Current Prices) Rs Lakh Sr
UTTARAKHAND Sectorwise Gross District Domestic Product (GDDP ) for the year : 2005-06 ( At Current Prices) Rs Lakh Sr. District Agricul- Forestry Fishing Mining & Manufa- Regi- Un Electricity, Constru- Trade,Hotels Railways No. Name ture & Logging Quarrying cturing stered regd. Gas & ction & Resta- MFG. MFG. MFG. W.supply urants 1 2 3 4 5 6 7 8 9 10 11 12 13 1 Uttarkashi 23523 3512 11 136 1495 111 1384 1280 8701 5600 0 2 Chamoli 29078 1752 25 1356 3326 256 3070 6869 16217 10214 134 3 Rudraprayag 9097 695 7 559 1199 205 994 514 9720 7458 384 4 Tehri Garhwal 37811 1080 8 9938 7940 4891 3049 3059 40788 24927 201 5 Dehradun 32657 6148 40 4897 16489 1255 15234 8649 72430 108870 7087 6 Garhwal 27110 2444 17 1836 16527 13332 3195 4020 23831 27803 393 7 Pithoragarh 29542 963 19 1143 4994 119 4875 2853 18667 15547 86 8 Bageshwar 12914 922 6 1527 1605 154 1451 749 8937 6521 67 9 Almora 51898 1002 11 549 5963 1681 4282 2420 22812 20160 460 10 Champawat 17192 4616 5 1551 2078 179 1899 981 6652 8814 67 11 Nainital 37345 12942 10 18250 22460 13298 9162 3672 29806 53824 6886 12 Udham Singh Nagar 82737 7606 541 1514 42652 23267 19385 2213 35013 73509 4575 13 Hardwar 92540 4058 353 5687 210124 186892 23232 3396 55220 90764 14732 Source: Directorate of Economics and Statistics,Govt. of Uttarakhand 1 of 8 UTTARAKHAND Sectorwise Gross District Domestic Product (GDDP ) for the year : 2005-06 ( At Current Prices) Rs Lakh Sr. -

Aditya Birla Fashion and Retail Launches `PEOPLE' in Haldwani For
Aditya Birla Fashion and Retail launches `PEOPLE’ in Haldwani for Young India ~The brand’s new fashion store in Haldwani, is spread over 5000sq.ft~ Haldwani, January 8th, 2017: Aditya Birla Fashion and Retail Ltd’s youth fashion brand, PEOPLE launched its first high street store in Haldwani at Nainital Road. Having established a strong affinity with fashionable Indians across 48 cities, PEOPLE is all set to ramp up the fashion quotient of youth with global fashion trends straight off the runway. Popular for its picturesque locales and rich heritage, Haldwani is a melting pot of diverse culture and offers an unmatched potential for youth-focused fashion brands. Commenting on the occasion, Mr. Sooraj Bhat, Chief Operating Officer, Fast Fashion Business, Aditya Birla Fashion and Retail Limited said, “Uttrakhand is an important market for us and we are excited to launch our first store in Haldwani. The scenic city has a burgeoning millennial base who loves experimenting with vibrant and vivid color palettes with bold patterns. The fantastic response from our consumers has encouraged us to explore new markets in metros and other cities. Our Haldwani store will offer the latest in women’s and men’s fashion.” PEOPLE’s brand new store spread over 5000 sq. ft., houses the newest styles which are both street perfect and college ready. Inspired by global runway trends, PEOPLE’s latest collection focuses on a diverse range of casual shirts, t-shirts, tops, kurtas, fusion tops, jeans, winter wear, and accessories, for both men and women. The newly launched Winter collection by PEOPLE is designed keeping in mind both comfort factor as well as latest trends for the youth who are always on the go. -

Ongoing Tender .Xlsx
Status of Ongoing Construction Works Dehradun Unit Cost Sanctioned SL.NO Name of Project (Rs. In Lac) Name Of Contractor 1 2 34 Construction of Rajiv Gandhi Navodaya 1 Vidhalaya Gairsain, Distt. Chamoli R.C. Bisht Haldwani (Package-1) 2093.10 Construction of Rajiv Gandhi Navodaya M/s Mahalaxmi Const. 2 Vidhalaya Gairsain, Distt. Chamoli Kashipuir (Package-2) Construction of Praposed Food 3 Comissioner office building at Ring 745.17 M/s Navya Associates Road Dehradun Construction of Praposed Food 4 Comissioner office building at Ring 154.31 M/s Sushil Prasad Road Dehradun (Balance work) Construction of uttrakhand space M/s Arihant 5 application center ( UCAC) at 494.45 Const.Dehradun Dehradun. Construction of District level 6 85.00 K.M. Traders Panchayati Resource Center at Tehri Construction of District level M/S Semwal 7 Panchayati Resource Center at 85.00 Construction Rudraprayag. Construction of Boundry wall of NTPC 8 391.91 Sri Balaji Enterprises. Towenship Joshimath. Construction of Proposed School 9 Building og GIC Bhori, Roorkee, 320.93 Arif Construction Haridwar. Construction of 50 Bedded Boys Hostel Ajay Chaudhary 10 at Govt. Poly. Vikas Nagar, Dehradun. 179.54 Builders Retrofitting, Strengthning & Renovation 11 66.05 Chamoli Associates of Ghanta Ghar, dehradun Construction of Work shop at Govt Poly 12 14.36 M/s Sushil Prasad Pitthowala, dehradun. Construction of Modle ITI Building at 13 190.90 M/s Arif Construction Jagjeetpur Haridwar, Uttarakhand Construction of Tin Shed for M/s Jasveer Singh 14 Uttarakhand Rajkiya Seva Chayan 9.50 Construciton Board, Distt.- Dehradun Construction of Proposed Doon Library M/s Jasveer Singh 15 & Research Centre at Dehradun 20.54 Construciton Construction of Govt. -

F. No. 10-6/2017-IA-Ill Government of India
F. No. 10-6/2017-IA-Ill Government of India Ministry of Environment, Forest and Climate Change (IA.III Section) Indira Paryavaran Bhawan, Jor Bagh Road, New Delhi - 3 Date: 10th October, 2017 To, Mukhya Nagar Adhikari Haldwani Nagar Nigam, Nagar Palika Parishad, Haldwani, District: Nainital - 263139, Uttarakhand E Mail: infoRnagarnigamhaldwani.com Subject: Integrated Municipal Solid Waste Management Project at Haldwani - Kathgodam, District Nainital, Uttarakhand by M/s Haldwani Nagar Nigam - Environmental Clearance - reg. Sir, This has reference to your online proposal No. IA/UK/MIS/62412/2015 dated 9th February 2017, submitted to this Ministry for grant of Environmental Clearance (EC) in terms of the provisions of the Environment Impact Assessment (EIA) Notification, 2006 under the Environment (Protection) Act, 1986. 2. The proposal for grant of environmental clearance to the project 'Integrated Municipal Solid Waste Management Project at Haldwani-Kathgodam, District Nainital, Uttarakhand promoted by M/s Haldwani Nagar Nigam' was considered by the Expert Appraisal Committee (Infra-2) in its meetings held on 12-14 April, 2017 and 21-24 August, 2017. The details of the project, as per the documents submitted by the project proponent, and also as informed during the above meeting, are under:- (i) The project involves Integrated Municipal Solid Waste Management Project at Haldwani- Kathgodam, District Nainital, Uttarakhand promoted by M/s Haldwani Nagar Nigam. (ii) As a part of the Jawaharlal Nehru National Urban Renewal Mission (JNNURM), Haldwani Nagar Nigam (HNN) has proposed treatment and disposal of MSW at Indira Nagar railway crossing on Sitarganj bypass, Haldwani. (iii) Integrated Municipal Solid Waste Management Facility has been taken up to cater the Haldwani City, Bhimtal, Kichha, Lalkuan and Rudrpur under administrative control of Haldwani Nagar Nigam. -

The Preparatory Survey for Uttarakhand Forest Resource Management Project in India
Japan International Cooperation Agency (JICA) Forest Department The State of Uttarakhand, India The Preparatory Survey for Uttarakhand Forest Resource Management Project in India Final Report Volume I I I: Attachment February 2014 NIPPON KOEI CO., LTD. JICA Pr eparatory Sur vey for Uttarakhand Forest Resource Management Project ATTACHMENT List of Attachment Attachment 2.2.1 Socio-economic Profile of Uttarakhand ................................................................................. 1 Attachment 2.7.1 Relevant Projects/ Programs on Watershed Management, Forestry Sector and Livelihood Improvement .......................................................................................................................... 4 Attachment 2.7.2 Map: Watershed Forest Projects ............................................................................................ 5 Attachment 2.7.3 List of Districts and Blocks covered by Watershed Management/ Livelihood Projects ........ 6 Attachment 2.7.4 List of Divisions covered by Forestry related Projects .......................................................... 7 Attachment 3.1.1 Map: Project Area Priority Ranges ........................................................................................ 8 Attachment 3.1.2 List of Recommended Priority Ranges and their District and Tehsil (Sub-District) .............. 9 Attachment 3.2.1 Map: Forest Crown Density Uttarakhand, 2011 .................................................................. 10 Attachment 3.2.2 Division-Wise Forest Cover ............................................................................................... -

Dr Bhuwan Chandra Melkani Dept of Commerce MB
BIO-DATA 1. Name and full correspondence address : Dr Bhuwan Chandra Melkani Dept of Commerce M. B. Govt. P. G. College, Haldwani (Nainital) Uttarakhand – 263139 2. E-mail and contact number(s) : [email protected] 9411161894(M) 3. Institution : M. B. Govt. P. G. College, Haldwani (Nainital) Uttarakhand 263139 4. Date of Birth: 28-03-1980 5. Academic Qualification (Undergraduate Onwards) S. Degree Year Subject University/Institution Division No. 1 B. Com 2000 Mgmt Group 1st Kumaun University, Nainital Second ,Accounting Group-2nd Bus, Eco and Business Law-3rd Group 2 M. Com 2002 Commerce(Accounting Kumaun University, Nainital Second Group) 3 Ph. D 2007 Commerce Kumaun University, Nainital - 6. Ph. D thesis title: Uttaranchal Main Zila Dudgh Utpadak Sahkari Sanghon Ki Karya Pranali Ka Mulyankan 7. Work experience 1 Contract Govt. Degree College, Jaiti, 25 Sep 2010 02 Jan Fixed Salary Lecturer Ramnagar & M.B.G.P.G 2017 College, Haldwani 2 Assistant M.B.G.P.G College, 02 Jan Still working 15600-39100 Professor Haldwani 2017 8. No. of Ph. D./M. Phil guided (Pursuing/Awarded Degree) S. No. Name of Student Title of Ph. D/M. Phil Year of Award 1. Anil Kumar - Pursuing 2. Tanuj - Pursuing 3. Vineet Pathak - Pursuing 4. Mukesh Upadhya - Pursuing 9. Published Books/Reports/Chapters/Articles etc.(Article published in ISBN only) S. Chapter Title/Paper Book Title Author’s Name Publisher Year with No. Title /Article/Journal ISBN Name 1 GST & Indian GST-A Road Map of Bhanu,Vinay 2018 Economy:Issues & Economic & Bhuwan Chandra Challenges Development for Melkani ISBN 978-93- (pg no- 8-13) New India 82972-25-9 2 ग्रामीण महिऱाओ के Vidyawarta Bhuwan Chandra Vidyawarta Vol-08, Issue सशक्तिकरण मे द嵍ु ध Melkani 15, 2016 핍यवसाय की भूममका (pg No- 153-157) ISSN 2319- 9318 3 महिऱाओ के सामाक्िक Research Journal of Bhuwan Chandra Madhya Pradesh 2010 एवं आ셍थिक ववकास मे Social & Life Melkani Vol-08,Year 04 महिऱा डरे ी की भूममका Sciences (pg No- 947-950) ISSN 0973- 3914 10. -

Water Equity and Tourism: a Case of Nainital District, Uttarakhand Yamini Yogya
Water Equity and Tourism: A Case of Nainital District, Uttarakhand Yamini Yogya Introduction This study is based on fieldwork that was carried out in the North Indian state of Uttarakhand over the course of a few years since 2014 under two projects. The first project was the Himalayan, Adaptation, Water and Resilience, where I was involved in the capacity of a research intern. The objective of the working package I was a part of aimed at exploring the impact of climate change on the livelihoods of communities living in the Upper Ganga Basin. The second project, carried out in the Kumaon region of Uttarakhand mapped the impact of multiple stressors (both climatic and non- climatic) on mountain farmers in two blocks of the Nainital district. This paper draws from both field experiences and data, but focusses primarily on observations and on- field narratives from Ramgarh and Dhari blocks of Nainital district in Uttarakhand. Urbanization and the increase in tourism infrastructure in the district over the past decade has resulted in a conflict over water access and equity. An increase in tourism operators in the region has led to a change in the social and cultural fabric of the norms that had been in place for decades. The development of tourism infrastructure has put an incredible amount of pressure on an already fragile mountain ecosystem, and necessitates the inequitable sharing of an extremely limited natural resource. At the village level, the local self-government or the Panchayat is tasked with dealing with conflicts that arise from the mis-appropriation of water. -

Draft Design and Monitoring Framework
Draft Design and Monitoring Framework Project Number: 38272 August 2011 IND: Uttarakhand Urban Sector Development Investment Program Tranche 2 A design and monitoring framework is an active document, progressively updated and revised as necessary, particularly following any changes in project design and implementation. In accordance with ADB’s public communications policy (2005), it is disclosed before appraisal of the project or program. This draft framework may change during processing of the project or program, and the revised version will be disclosed as an appendix to the report and recommendation of the President. DESIGN AND MONITORING FRAMEWORK FOR PROJECT 2 Performance Targets and Data Sources and Assumptions Design Summary Indicators Reporting Mechanisms and Risks Impact Assumptions People, especially Pressured water supply Water supply and State government or vulnerable household,a will hours increased from 2-8 sewerage operators’ or regulator regularly revises have increased access to hours per day in 2007 to 24 third party validators’ the user charges on time. better quality and hours in 2016. service quality compliance Government financed sustainable urban Centralized sewerage reports. water supply works in infrastructure and services systems’ sewage collection Dehradun, Nainital, in 31 urban towns. from household under Haldwani, and Haridwar (synchronized with MFF’s increased from 0% in 2007 completes on time. DMF outcome) to 60% of households in UDD implements SWM, 2016. urban road, slum- Coverage of regular daily ULBs’ and waste upgrading subprojects household waste collection collection operators’ under projects 3 and 4. increased from 0% to 72% reports on household Risks of households in 2016. waste collection. Power supply to WTP, Vehicle travel time per PWD and ULB’s road STP and pumping stations kilometer reduced from [] in conditions surveys. -
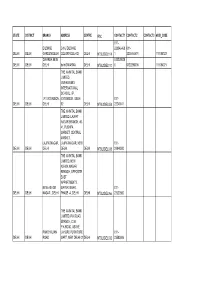
State District Branch Address Centre Ifsc
STATE DISTRICT BRANCH ADDRESS CENTRE IFSC CONTACT1 CONTACT2 CONTACT3 MICR_CODE 011- DILSHAD C-16, DILSHAD 223546460 011- DELHI DELHI GARDEN DELHI COLONY DELHIQ DELHI NTBL0DEL114 1 2235464601 110184023 DWARKA NEW 512228030 DELHI DELHI DELHI delhi DWARKA DELHI NTBL0DEL110 0 5122280300 110184021 THE NAINITAL BANK LIMITED, VIVEKANAND INTERNATIONAL SCHOOL, I.P. I.P. EXTENSION, EXTENSION, DELHI 011- DELHI DELHI DELHI 92 DELHI NTBL0DEL053 22240041 THE NAINITAL BANK LIMITED, LAJPAT NAGAR BRANCH, 40- 41, PUSHPA MARKET, CENTRAL MARKET, LAJPATNAGAR, LAJPATNAGAR, NEW 011- DELHI DELHI DELHI DELHI DELHI NTBL0DEL038 29848500 THE NAINITAL BANK LIMITED, NEW ASHOK NAGAR BRANCH, OPPOSITE EAST APPARTMENTS, NEW ASHOK MAYUR VIHAR, 011- DELHI DELHI NAGAR , DELHI PHASE -A, DELHI DELHI NTBL0DEL066 22622800 THE NAINITAL BANK LIMITED, P.K.ROAD BRANCH, C-36, P.K.ROAD, ABOVE PANCHKUIAN LAHORE FURNITURE 011- DELHI DELHI ROAD MART, NEW DELHI 01 DELHI NTBL0DEL032 23583606 THE NAINITAL BANK LIMITED, PAPPANKALAN BRANCH, 29/2, VIJAY ENCLAVE, PALAM PAPPANKALAN( DABRI ROAD, KAPAS 011- DELHI DELHI DWARKA) DWARKA, DELHI HERA NTBL0DEL059 25055006 THE NAINITAL BANK LIMITED, PATPARGANJ BRANCH, P-37, PANDAV NAGAR, 011- DELHI DELHI PATPARGANJ PATPARGANJ, DELHI DELHI NTBL0DEL047 22750529 THE NAINITAL BANK LIMITED, PITAMPURA BRANCH, SATABDI HOUSE, PLOTNO. 3, COMMERCIAL COMPLEX, ROHIT KUNJ, WEST PITAMPURA , PITAMPURA 110034, 011- DELHI DELHI DELHI DELHI DELHI NTBL0DEL049 27353273 THE NAINITAL BANK LIMITED, ROHINI BRANCH, E-4, SECTOR 16, JAIN BHARTI MODEL, PUBLIC SCHOOL, ROHINI, ROHINI, -

LIST of EMPANELLED HOSPITALS UNDER U-HEALTH Neurology
LIST OF EMPANELLED HOSPITALS UNDER U-HEALTH Neurology S.No Hospital Name Location Specialization Address Contact Person Contact Number Mr. Sarveshresth Gupta / 1 1 Max Hospital Dehradun Cardiology/Neurology/Ortho Rajpur Road, Dehradun 9997399111/9760462288 Mr. P.N. Tripathi Heart 2 1 Fortis Escorts Hospital Dehradun Heart 2 nd Floor Coronation Hospital Mr.Vipin Bahuguna 7895670999/ 0135-3980201 Cardiology/Neurology/Urology/Nephro 3 2 Metro Hospitals & Heart Institutes Haridwar Plot No- F-1, Sec 6-A,SIDCUL, Haridwar,UK Mr. Ankit Negi 8191902613/9997013383 /Gen.Sugery 4 3 Bharat Heart Institute Dehradun Heart 55,East Canal Road,Dehradun Dr.Javed 7520099155 5 4 National Heart Institute New Delhi Heart 49-50 Community Centre, East of Kailash, New Delhi Mr.R B S Rawat 08527537508 Eye 6 1 Nirmal Ashram Eye Institute Dehradun Eye Khairi Kalan, P.O.- Satyanarayan, Near Nepali Farm Mr. Waseem/Ms Harmeet 9927177205/8191030003 7 2 Navjyoti Eye Hospital Dehradun Eye Nehru Colony, Dehradun Mr. Lokendra 9198900638 8 3 Drishti Eye Centre Dehradun Eye 58,Chakrata Road,Dehradun Dr.Chirag 9358100350 9 4 Singh Eye Hospital Dehradun Eye 230, Araghar Chowk, HaridwarRoad,Dehradun Dr. Amit singh 9412347530 10 5 Eye Q Hospital Roorkee Eye Chanderpuri, Near Sindhi Sweats, Roorkee Mr. Bisht 8958355706 11 6 The Eye Clinic Dehradun Eye 3-A Chakrata Road, Near Doon Paramedical Dr.Subha Nagesh 8954948708 Prakash Eye Hospital & Lazer 12 7 Rudrapur Eye Cicil Lines, Doctor's Colony, Rudrapur Dr. Sarika Garg Centre 13 8 Jeevan Jyoti Clinic Dehradun Eye 21,C-19A, Turner Road, Clement Town, Dehradun Dr. Smita Mehra 7895715773 34/2, Special Wing, Near Amitabh Textile Mill Pump House, Kendriya 14 9 Ramrati Eye Hospital Dehradun Eye Dr. -

Comprehensive Mobility Plan for Dehradun
COMPREHENSIVE MOBILITY PLAN FOR DEHRADUN - RISHIKESH – HARIDWAR METROPOLITAN AREA May 2019 Comprehensive Mobility Plan For Dehradun - Rishikesh – Haridwar Metropolitan Area Quality Management Report Prepared Report Report Revision Date Remarks By Reviewed By Approved By 2018 1 Ankush Malhotra Yashi Tandon Mahesh Chenna S.Ramakrishna N.Sheshadri 10/09/2018 Neetu Joseph (Project Head) (Reviewer) Nishant Gaikwad Midhun Sankar Mahesh Chenna Neetu Joseph Nishant Gaikwad S.Ramakrishna N.Sheshadri 2 28/05/2019 Hemanga Ranjan (Project Head) (Reviewer) Goswami Angel Joseph TABLE OF CONTENTS Comprehensive Mobility Plan for Metropolitan Area focusing Dehradun-Haridwar-Rishikesh TABLE OF CONTENTS EXECUTIVE SUMARY...........................................................................................i 1 1 INTRODUCTION .................................................................................................................. 14 1.1 Study Background ......................................................................................................................... 14 1.2 Need for Comprehensive Mobility Plan ........................................................................................ 15 1.3 Objectives and Scope of the Study ................................................................................................ 16 1.4 Study Area Definition .................................................................................................................... 19 1.5 Structure of the Report ................................................................................................................ -

Rudrapur and Haldwani Risk Profile
Strategic Plan for Risk Reduction: Rudrapur & Haldwani August 2018 STATE LEVEL ENDORSEMENT “The magnitude of hazards and frequency of extreme weather events in Uttarakhand has increased due to climate change. The traditional methods of disaster management need to be overhauled, earlier the traditional methods used to be relief, response and rehabilitation, but now the whole scenario has changed. We really have to upgrade our capacities and strengthen our people.” Mr. Amit Singh Negi Disaster Management Secretary, Govt. of Uttarakhand (State Workshop on “Strengthening Resilience to Climate Change Related Disaster Risks” held in Dehradun on 21st July 2017) Uttarakhand Disaster Recovery Project Page i Strategic Plan for Risk Reduction: Rudrapur & Haldwani August 2018 Table of Contents 1 Introduction............................................................................................................................................................................................................... 1 1.1 Overview of the Area ......................................................................................................................................................................................... 1 1.2 About this Strategic Plan ................................................................................................................................................................................... 3 1.3 Area and Community Profile .............................................................................................................................................................................