Cytopathology Pathology
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Medical Directors Arup Medical Directors and Consulting Faculty | 2015
MEDICAL DIRECTORS ARUP MEDICAL DIRECTORS AND CONSULTING FACULTY | 2015 MAY 2015 www.aruplab.com Information in this brochure is current as of May 2015. All content is subject to change. Please contact ARUP Client Services at (800) 522-2787 with any questions or concerns. ARUP LABORATORIES ARUP Laboratories is a national clinical and anatomic pathology reference laboratory and a nonprofit enterprise of the University of Utah and its Department of Pathology. Located in Salt Lake City, Utah, ARUP offers in excess of 3,000 tests and test combinations, ranging from routine screening tests to esoteric molecular and genetic assays. Rather than competing with its clients for physician office business, ARUP chooses instead to support clients’ existing test menus by offering complex and unique tests, with accompanying consultative support, to enhance their abilities to provide local and regional laboratory services. ARUP’s clients include many of the nation’s university teaching hospitals and children’s hospitals, as well as multihospital groups, major commercial laboratories, group purchasing organizations, military and other government facilities, and major clinics. In addition, ARUP is a worldwide leader in innovative laboratory research and development, led by the efforts of the ARUP Institute for Clinical and Experimental Pathology®. Since its formation in 1984 by the Department of Pathology at the University of Utah, ARUP has founded its reputation on reliable and consistent laboratory testing and service. This simple strategy contributes significantly to client satisfaction. When ARUP conducts surveys, clients regularly rate ARUP highly and respond that they would recommend ARUP to others. As the most responsive source of quality information and knowledge, ARUP strives to be the reference laboratory of choice for community healthcare systems. -

Clinical Microbiology Core Rotation
C:\Documents and Settings\jdnoll\Desktop\UofFCytolRot10.doc CYTOPATHOLOGY ROTATION Site: University of Florida-Gainesville/ Shands North & South Tower Faculty: Peter Drew, M.D. (4-4969) Larry J. Fowler, M.D., Cytology Director (4-4959) Jacquelyn Knapik, M.D. (4-3887) Li Lu, M.D, PhD (63-6440) Demaretta Rush, M.D. (5-3364) Edward Wilkinson, M.D. (4-4962) Anthony Yachnis, M.D., Anatomic Pathology Director (4-4951) Current Cytopathology Fellow Charlene M. Lewis, CT (ASCP) Superviser Deborah A. Carroll, CT, (ASCP) Patricia Christensen, CT (ASCP) David A. Lemire, CT (ASCP) Rotation Periods: 12 weeks for CORE Rotation broken into three 4 week rotations (a portion of the 12 weeks may be on the VA Hospital Cytology Rotation). Offered all 12 months of any training year. I. General Organization: The cytopathology core rotation consists of three 4 week rotations (total 12 weeks) routinely placed within the 1st thru 4th PGY training years of a 4 year APCP training program. The CORE rotation may also be done in one combined 12 week rotation upon special request. Cervical biopsies and local gynecologic excisions are signed out in tandem with cytology specimens (correlation of surgical and cytologic specimens). The first 4 week's rotation, residents review the day's cases at the time of sign-out with the faculty member on service. During the second and third 4 week rotations residents preview cases before sign-out, render preliminary interpretations, and receive feedback at sign out (graduated responsibility). Fellows preview the slides before sign-out and render interpretations, and have the option to attend sign-out or receive feedback from the attending pathologist later (graduated responsibility). -

Cytopathology Fellowship Faculty of Medicine, Postgraduate Medical Education
Cytopathology Fellowship Faculty of Medicine, Postgraduate Medical Education Institution: McGill University and McGill University Health Centre Location: Department of Pathology, MUHC and Duff Medical Building Type of Fellowship: One year clinical fellowship in Cytopathology Fellowship Program Director: Dr. Manon Auger Program Information Number of fellowship positions requested: One Academic affiliation: McGill University Name of hospitals involved in training: McGill University Health Centre (MUHC) Background: The fellowship will be principally based at the MUHC. This one-year post-residency clinical fellowship offers a comprehensive, integrated exposure to the field of Cytopathology. The cytological specimens handled at the MUHC include approximately 50,000 gynecological specimens per year (including approximately 7000 colposcopic specimens) as well as 10,000 non-gynecological specimens (including approximately 4000 fine needle aspirates). Exposure to liquid- based cytology is provided. Research activity and publications: The fellow will be expected to participate in at least one major research project involving, at minimum, a review of a series of cases (i.e., not simply a case report) with a view to publication in a peer-reviewed journal (i.e. not only presentation at a meeting). In addition, other research projects may be carried out, including case reports. Mission and objectives: The general mission of the fellowship is to provide training in the interpretation of cytopathology with a view to learn the entire spectrum of reactive -

Chlamydia Trachomatis Infection Is Driven by Nonprotective Immune Cells That Are Distinct from Protective Populations
Pathology after Chlamydia trachomatis infection is driven by nonprotective immune cells that are distinct from protective populations Rebeccah S. Lijeka,b,1, Jennifer D. Helblea, Andrew J. Olivea,c, Kyra W. Seigerb, and Michael N. Starnbacha,1 aDepartment of Microbiology and Immunobiology, Harvard Medical School, Boston, MA 02115; bDepartment of Biological Sciences, Mount Holyoke College, South Hadley, MA 01075; and cDepartment of Microbiology and Physiological Systems, University of Massachusetts Medical School, Worcester, MA 01605 Edited by Rafi Ahmed, Emory University, Atlanta, GA, and approved December 27, 2017 (received for review June 23, 2017) Infection with Chlamydia trachomatis drives severe mucosal immu- sequence identity, Chlamydia muridarum, the extent to which the nopathology; however, the immune responses that are required for molecular pathogenesis of C. muridarum represents that of Ct is mediating pathology vs. protection are not well understood. Here, unknown (6). Ct serovar L2 (Ct L2) is capable of infecting the we employed a mouse model to identify immune responses re- mouse upper genital tract when inoculated across the cervix into quired for C. trachomatis-induced upper genital tract pathology the uterus (7, 8) but it does not induce robust immunopathology. and to determine whether these responses are also required for This is consistent with the human disease phenotype caused by Ct L2, bacterial clearance. In mice as in humans, immunopathology was which disseminates to the lymph nodes causing lymphogranuloma characterized by extravasation of leukocytes into the upper genital venereum (LGV) and is not a major cause of mucosal immunopa- thology in the female upper genital tract (uterus and ovaries). tract that occluded luminal spaces in the uterus and ovaries. -

Department of Experimental Pathology, Immunology and Microbiology 531
Department of Experimental Pathology, Immunology and Microbiology 531 Department of Experimental Pathology, Immunology and Microbiology Interim Chairperson: Zaatari, Ghazi Vice Chairperson: Matar, Ghassan Professors: Abdelnoor, Alexander; Khouri, Samia; Matar, Ghassan; Sayegh, Mohamed; Zaatari, Ghazi Associate Professor: Rahal, Elias Assistant Professors: Al-Awar, Ghassan; El Hajj, Hiba; Shirinian, Margret; Zaraket, Hassan The Department of Experimental Pathology, Immunology and Microbiology offers courses to medical laboratory sciences (MLSP) students as well as nursing, medical, and graduate students. It offers a graduate program (discipline of Microbiology and Immunology) leading to a master’s degree (MS) or doctoral degree (PhD) in Biomedical sciences. The requirements for admission to the graduate program are stated on page 33 of this catalogue. IDTH 203 The immune System in Health and Disease 37.28; 3 cr. See Interdepartmental Courses. IDTH 205 Microbiology and Infectious Diseases 37.28; 5 cr. See Interdepartmental Courses. MBIM 223 Parasitology for MLSP Students 39.39; 4 cr. Second semester. MBIM 237 Microbiology and Immunology for Nursing Degree Students 32.64; 3 cr. A course on the fundamental aspects of medical microbiology and immunology for nursing students. Second semester. MBIM 260 Elective in Infectious Diseases for Medicine III and IV 0.180 A course on basic evaluation, diagnosis, and management of infectious diseases. One month. MBIM 261 Elective in Immunology for Medicine III and IV 0.180 A course that is an introduction to immunological research and its application to clinical practice. One month. MBIM 310 Basic Immunology 32.32; 3 cr. A course on innate and adaptive immune mechanisms, infection and immunity, vaccination, immune mechanisms in tissue injury and therapeutic immunology. -

Deciphering the Triad of Infection, Immunity and Pathology
INSIGHT DISEASE Deciphering the triad of infection, immunity and pathology The factors which drive and control disease progression can be inferred from mathematical models that integrate measures of immune responses, data from tissue sampling and markers of infection dynamics. FREDERIK GRAW immune actors in the body. Now, in eLife, Related research article Myers MA, Smith Amber Smith and colleagues at St. Jude Child- AP, Lane LC, Moquin DJ, Aogo R, Woolard ren’s Research Hospital, the University of Ten- S, Thomas P, Vogel P, Smith AM. 2021. nessee Health Science Center and the Dynamically linking influenza virus infection Washington University School of Medicine – kinetics, lung injury, inflammation, and dis- including Margaret Myers and Amanda Smith as ease severity. eLife 10:e68864. doi: 10. joing first authors – report how viral infection, 7554/eLife.68864 counteracting immune responses and lung pathology interact as mice fight off influenza A (Myers et al., 2021). First, the team tracked how viral load and the number of CD8+ T cells, an important immune fever, a cough, a splitting headache... actor that helps to clear infected cells, pro- Being sick often comes with tell-tale gressed over time. In combination with mathe- A signs which worsen as the disease pro- matical models, these measurements allowed gresses and tissues become damaged. These Myers et al. to estimate several parameters that symptoms result from complex interactions reflect the pace at which the virus replicates, the between the infecting pathogen, the inflamma- strength of the immune response, and the inter- tion process, and the response from the immune actions between these processes. -

Overview of Pathology and Its Related Disciplines - Soheir Mahmoud Mahfouz
MEDICAL SCIENCES – Vol.I -Overview of Pathology and its Related Disciplines - Soheir Mahmoud Mahfouz OVERVIEW OF PATHOLOGY AND ITS RELATED DISCIPLINES Soheir Mahmoud Mahfouz Cairo University, Kasr El Ainy Hospital, Egypt Keywords: Pathology, Pathology disciplines, Pathology techniques, Ancillary diagnostic methods, General Pathology, Special Pathology Contents 1. Introduction 1.1 Pathology coverage 1.1.1 Etiology and Pathogenesis of a Disease 1.1.2 Manifestations of Disease (Lesions) 1.1.3 Phases Of A Disease Process (Course) 1.2 Physician’s approach to patient 1.3 Types of pathologists and affiliated specialties 1.4 Role of pathologist 2. Pathology and its related disciplines 2.1 Cytology 2.1.1 Cytology Samples 2.1.2 Technical Aspects 2.1.3 Examination of Sample and Diagnosis 3. Pathology techniques and ancillary diagnostic methods 3.1 Macroscopic pathology 3.2 Light Microscopy 3.3 Polarizing light microscopy 3.4 Electron microscopy (EM) 3.5 Confocal Microscopy 3.6 Frozen section 3.7 Cyto/histochemistry 3.8 Immunocyto/histochemical methods 3.9 Molecular and genetic methods of diagnosis 3.10 Quantitative methods 4. Types of tests used in pathology 4.1 DiagnosticUNESCO tests – EOLSS 4.2 Quantitative tests 4.3 Prognostic tests 5. The scope of SAMPLEpathology & its main divisions CHAPTERS 6. Conclusions Glossary Bibliography Biographical sketch Summary Pathology is the science of disease. It deals with deviations from normal body function and ©Encyclopedia of Life Support Systems (EOLSS) MEDICAL SCIENCES – Vol.I -Overview of Pathology and its Related Disciplines - Soheir Mahmoud Mahfouz structure. Many disciplines are involved in the study of disease, as it is necessary to understand the complex causes and effects of various disorders that affect the organs and body as a whole. -

The Papanicolaou and Frost 2019 Cytopathology Tutorial
Y 5 E Coun cil for Continuing Medical Education to provide continuing N 1 Statement of Need , D G . T N medical education for physicians. A Diagnostic cytopathology per forms a vital role in the O T S W N D S I di agnosis and treatment of non-neoplastic and neoplastic T Weill Cornell Medicine designates this live activity for a O T A O I R T 15. 0 1 P AMA PRA Category Credi t(s )™. P le sions. This course is intended to advance and develop S M maximum of H . R T R S I the knowledge base of pathologists in cytopathology. P Physicians should claim only the credit commensurate with E M U P Current criteria and concepts based on cytomorphologic the extent of their participation in the activity. S evaluation and approaches to differential diagnosis are the core need for the practice of cytopathology. This course is Maintenance of Certification designed to provide a practical update to cytopathologists, The American Board of Pathology has approved the Tutorial cytotechnologists, general pathologists and fellows/residents as a Self- Assessment Module (SAM) for Part II of the ABP in training. Main tenance of Certifica tion (MOC) program and designates this edu cational activity for a max imum of 10.0 SAM Course Goals and Objectives Credit(s). Physi cians should claim only the credit commen - This CME approved course will lead to improved practice surate with the extent of their participation in the activity. of cytopathology leading to enhanced pa tient care. At the conclusion of this course the at tendees will be able to: Disclosure of Relationships/ Content Validity • Evaluate cytologic criteria of various non-neoplastic It is the policy of Weill Cornell Medicine to adhere to and neoplastic lesions. -
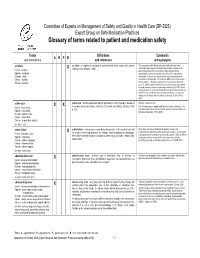
Glossary of Terms Related to Patient and Medication Safety
Committee of Experts on Management of Safety and Quality in Health Care (SP-SQS) Expert Group on Safe Medication Practices Glossary of terms related to patient and medication safety Terms Definitions Comments A R P B and translations and references and synonyms accident accident : an unplanned, unexpected, and undesired event, usually with adverse “For many years safety officials and public health authorities have Xconsequences (Senders, 1994). discouraged use of the word "accident" when it refers to injuries or the French : accident events that produce them. An accident is often understood to be Spanish : accidente unpredictable -a chance occurrence or an "act of God"- and therefore German : Unfall unavoidable. However, most injuries and their precipitating events are Italiano : incidente predictable and preventable. That is why the BMJ has decided to ban the Slovene : nesreča word accident. (…) Purging a common term from our lexicon will not be easy. "Accident" remains entrenched in lay and medical discourse and will no doubt continue to appear in manuscripts submitted to the BMJ. We are asking our editors to be vigilant in detecting and rejecting inappropriate use of the "A" word, and we trust that our readers will keep us on our toes by alerting us to instances when "accidents" slip through.” (Davis & Pless, 2001) active error X X active error : an error associated with the performance of the ‘front-line’ operator of Synonym : sharp-end error French : erreur active a complex system and whose effects are felt almost immediately. (Reason, 1990, This definition has been slightly modified by the Institute of Medicine : “an p.173) error that occurs at the level of the frontline operator and whose effects are Spanish : error activo felt almost immediately.” (Kohn, 2000) German : aktiver Fehler Italiano : errore attivo Slovene : neposredna napaka see also : error active failure active failures : actions or processes during the provision of direct patient care that Since failure is a term not defined in the glossary, its use is not X recommended. -

Anesthesiology
Anesthesiology RESIDENCY PROGRAM AT Ocala Regional Medical Center Welcome to the AboutAnesthesiology HCA Healthcare Residency Program at Ocala Regional Medical Center The Anesthesiology Residency Program at Ocala Regional Medical Center is part of the HCA Healthcare Graduate Medical Education network. HCA Healthcare is the nation’s leading provider of quality, patient-centered care. We are also the leader in graduate medical education, all brought together by a single mission: Above all else, we are committed to the care and improvement of human life. ACGME ID: 401100212 Tracks & Positions Tracks NRMP # Available Positions Advanced 1587040A0 9 Salary PGY2 PGY3 PGY4 $55,456 $58,590 $60,630 Welcome and thank you for your interest in our program. In partnership with HCA Healthcare GME, we adhere to the mission statement, “Above all else, we are committed to the care and improvement of human life”. The Anesthesiology Residency Program is committed to training physicians in the science and practice of anesthesiology and perioperative medicine. In this context, we will train our colleagues in delivering evidence-based, high quality medical care emphasizing patient safety goals, and in which patient and family satisfaction is optimized. Excellence, collegiality, compassion, and integrity in patient care are the core tenets of our program education. We envision the residency program as a safe, positive and inspiring clinical environment to improve health outcomes and better serve our community. Sincerely, Ettore Crimi, MD Program Director Our faculty Ettore Crimi MD, Program Director • Anesthesiology Residency completed at MGH/Harvard • Cardiothoracic Fellowship completed at Stanford • Critical Care Fellowship completed at University of Florida • Subspecialty interests include critical care medicine, cardiothoracic anesthesia, and translational research. -

2021 Anatomic & Clinical Pathology
BEAUMONT LABORATORY 2021 ANATOMIC & CLINICAL PATHOLOGY Physician Biographies Expertise BEAUMONT LABORATORY • 800-551-0488 BEAUMONT LABORATORY ANATOMIC & CLINICAL PATHOLOGY • PHYSICIAN BIOGRAPHIES Peter Millward, M.D. Mitual Amin, M.D. Chief of Clinical Pathology, Beaumont Health Interim Chair, Pathology and Laboratory Medicine, Interim Chief of Pathology Service Line, Beaumont Health Royal Oak Interim Physician Executive, Beaumont Medical Group Interim Chair, Department of Pathology and Laboratory Medicine, Oakland University William Beaumont School Interim System Medical Director, Beaumont Laboratory of Medicine Outreach Services Board certification Associate Medical Director, Blood Bank and • Anatomic and Clinical Pathology, Transfusion Medicine, Beaumont Health American Board of Pathology Board certification Additional fellowship training • Anatomic and Clinical Pathology, • Surgical Pathology American Board of Pathology Special interests Subspecialty board certification • Breast Pathology, Genitourinary Pathology, • Blood Banking and Transfusion Medicine, Gastrointestinal Pathology American Board of Pathology Lubna Alattia, M.D. Kurt D. Bernacki, M.D. Cytopathologist and Surgical Pathologist, Trenton System Medical Director, Surgical Pathology Board certification Beaumont Health • Anatomic and Clinical Pathology, Chief, Pathology Laboratory, West Bloomfield American Board of Pathology Breast Care Center Subspecialty board certification Diagnostic Lead, Pulmonary Tumor Pathology • Cytopathology, American Board of Pathology Diagnostic -

ASC and PSC Position Statement on Use of Molecular
American Society of Cytopathology (ASC) and Papanicolaou Society of Cytopathology (PSC) Joint Position Statement on The Use of Molecular Testing on Cytologic Specimens Cytomorphologic evaluation of exfoliative and aspiration cytology specimens play an essential role in guiding the management of patients undergoing evaluation for neoplasia. In recent years, medicine has witnessed an explosive growth in our recognition and understanding of molecular genetic abnormalities that drive the development and progression of various cancers, as well as the therapeutic relevance of these abnormalities which mandates that molecular testing be performed for management decisions. Technologic advances that enable minimally invasive interventional modalities to obtain tissue for diagnosis have resulted in the increasing importance of cytologic specimens for patient management. Therefore, judicious and effective integration of molecular ancillary testing with cytopathology specimen acquisition, processing, and analysis is required in order to ensure timely and appropriate patient management. Molecular studies can serve as diagnostic adjuncts to aid in cytomorphologic classification and/or to interrogate specific relevant biomarkers, thereby providing information pertinent to prognosis and targeted therapy. Salient examples illustrating the intimate interface between cytopathology and molecular diagnostics include: (1) human papillomavirus testing on cervical cytology specimens and metastatic and primary head and neck cancers; (2) mutation panel and gene expression classifier testing on indeterminate thyroid fine needle aspirates (FNAs); (3) EGFR/ALK/ROS1 analysis on advanced‐stage lung adenocarcinoma; (4) BRAF mutational analysis in melanoma and thyroid cancer FNAs; (5) fluorescence in‐situ hybridization (FISH) testing applied to a variety of specimens such as urinary tract cytology, pancreatobiliary brushings, effusions, and cytologic samples from sarcomas and lymphomas; and (6) the application of next generation sequencing (NGS) to cytologic specimens.