PDF Generation Date :- Tue, 07 Sep 2021 19:52:03
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Imarat Shariah Bihar Patna
IMARAT SHARIAH PHULWARI SHARIF, PATNA For relief and rehabilitation Bihar floods 2017 Emergency Relief Appeal Flood Effected areas of Bihar Respected Brother in Islam Assalamo Alaikum Warahmatullah May Allah bestow his glorious upon you and your family! Indeed you know about the devastating flood in Bihar. Heavy rainfall in Bihar led to the overflow of major rivers like Mahananda, Koshi and Kankai that caused flooding in several districts in Bihar. The floods have washed away thousands of huts, badly damaged buildings, roads, bridges and standing crops worth crores. Kishanganj, Araria, Purnea and Katihar are worst-hit by the floods where thousands have been displaced. About 14.3 million people in Bihar have been affected as per the government reports across eighteen districts. Several towns of Araria, Purnia, Kishanganj and Katihar districts are witnessing unprecedented levels of water. Rail connection has been disrupted. some Railway stations are flooded. Address: Imarat Shariah At+P.O. Phulwari Sharif, Distt. Patna, Bihar-801505 (India) Tel.: 0612-2555668/2555351/2555280(Fax) E-mail: [email protected], website:www.imaratshariah.com A/c Name IMARAT SHARIAH: A/c No.10331726226 STATE BANK OF INDIA. JUDGES COURT ROAD, PATNA, IFSC Bank Code: SBIN0001233 IMARAT SHARIAH PHULWARI SHARIF, PATNA For relief and rehabilitation Many villages have lost road connections. Army and IAF teams have joined the NDRF team to rescue people and help with the crisis. Lakhs of people are stranded and have taken shelter on canals, school buildings and places of higher altitude. People were not forewarned and that has made the situation worse. The cause of the floods is heavy rains in the catchment area of several rivers that flow in this region (catchment area is in Nepal) and that causing breach in embankments of rivers and canals. -

Of India 100935 Parampara Foundation Hanumant Nagar ,Ward No
AO AO Name Address Block District Mobile Email Code Number 97634 Chandra Rekha Shivpuri Shiv Mandir Road Ward No 09 Araria Araria 9661056042 [email protected] Development Foundation Araria Araria 97500 Divya Dristi Bharat Divya Dristi Bharat Chitragupt Araria Araria 9304004533 [email protected] Nagar,Ward No-21,Near Subhash Stadium,Araria 854311 Bihar Araria 100340 Maxwell Computer Centre Hanumant Nagar, Ward No 15, Ashram Araria Araria 9934606071 [email protected] Road Araria 98667 National Harmony Work & Hanumant Nagar, Ward No.-15, Po+Ps- Araria Araria 9973299101 [email protected] Welfare Development Araria, Bihar Araria Organisation Of India 100935 Parampara Foundation Hanumant Nagar ,Ward No. 16,Near Araria Araria 7644088124 [email protected] Durga Mandir Araria 97613 Sarthak Foundation C/O - Taranand Mishra , Shivpuri Ward Araria Araria 8757872102 [email protected] No. 09 P.O + P.S - Araria Araria 98590 Vivekanand Institute Of 1st Floor Milan Market Infront Of Canara Araria Araria 9955312121 [email protected] Information Technology Bank Near Adb Chowk Bus Stand Road Araria Araria 100610 Ambedkar Seva Sansthan, Joyprakashnagar Wardno-7 Shivpuri Araria Araria 8863024705 [email protected] C/O-Krishnamaya Institute Joyprakash Nagar Ward No -7 Araria Of Higher Education 99468 Prerna Society Of Khajuri Bazar Araria Bharga Araria 7835050423 [email protected] Technical Education And ma Research 100101 Youth Forum Forbesganj Bharga Araria 7764868759 [email protected] -

State District Name of Bank Bank Branch/ Financial Literacy Centre
State District Name of Bank Branch/ Address ITI Code ITI Name ITI Address State District Phone Email Bank Financial Category Number Literacy Centre Bihar Araria State Araria Lead Bank Office, PR10000055 Al-Sahaba Industrial P Alamtala Forbesganj Bihar Araria NULL Bank of ADB Building, Training Institute India Araria, Pin- 854311 Bihar Arwal PNB ARWAL ARWAL PR10000083 Adarsh ITC P Umerabad Bihar Arwal NULL Bihar Arwal PNB ARWAL ARWAL PR10000284 Shakuntalam ITC P Prasadi English Bihar Arwal NULL Bihar Arwal PNB ARWAL ARWAL PR10000346 Aditya ITC P At. Wasilpur, Main Road, Bihar Arwal NULL P.O. Arwal, Bihar Arwal PNB ARWAL ARWAL PR10000396 Vikramshila Private P At. Rojapar, P.O. Arwal Bihar Arwal NULL ITI Bihar Arwal PNB ARWAL ARWAL PR10000652 Ram Bhaman Singh P At-Purani Bazar P.o+P.S- Bihar Arwal NULL Private ITI Arwal Bihar Arwal PNB ARWAL ARWAL PR10000677 Sukhdeo Institute Of P Kurtha, Arwal Bihar Arwal NULL Tecnology Private ITI, Bihar Arwal PNB ARWAL ARWAL PR10000707 Dr. Rajendra Prasad P Mubarkpur, Kurtha Arwal Bihar Arwal NULL Private ITI, Bihar Aurangabad PUNJAB DAUDNAGAR DAUDNAGAR PR10000027 New Sai Private ITI- P Aurangabad Road, Bihar Aurangabad NULL NATIONA Bhakharuan More, , Tehsil- L BANK Daudnagar , , Aurangabad - 824113 Bihar Aurangabad PUNJAB AURANGABAD AURANGABAD PR10000064 Adharsh Industrial P Josai More Udyog Bihar Aurangabad NULL NATIONA Training Centre Pradhikar Campus L BANK Bihar Aurangabad MADHYA DAUDNAGAR DAUDNAGAR PR10000108 Sardar Vallabh Bhai P Daudnagar Bihar Aurangabad NULL BIHAR Patel ITC, Daudnagar GRAMIN BANK Bihar Aurangabad MADHYA DAUDNAGAR DAUDNAGAR PR10000142 Adarsh ITC, P AT-,Growth centre ,Jasoia Bihar Aurangabad NULL BIHAR Daudnagar More Daudnagar GRAMIN BANK Bihar Aurangabad PUNJAB RATANUA RATANUA PR10000196 Progresive ITC P At-Growth Center Josia Bihar Aurangabad NULL NATIONA More L BANK Bihar Aurangabad MADHYA DAUDNAGAR DAUDNAGAR PR10000199 Arya Bhatt ITC P Patel Nagar, Daud Nagar Bihar Aurangabad NULL BIHAR GRAMIN BANK Bihar Aurangabad PUNJAB OLD GT RD. -
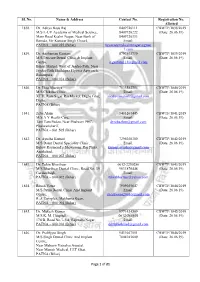
Cbwtf/1838/2019
Sl. No. Name & Address Contact No. Registration No. Allotted 1838. Dr. Aditya Basu Raj 8409726111 CBWTF/1838/2019 M/S L-UV Academy of Medical Science, 8409726222 (Date: 26.06.19) Main Road Keshri Nagar, Near Bank of 8409726333 Baroda, Vir Kunwar Singh Chowk, Email: PATNA – 800 025 (Bihar) luvacademykeshrinagar@gmai l.com 1839. Dr. Anshuman Gautam 8797503739 CBWTF/1839/2019 M/S Pericare Dental Clinic & Implant Email: (Date: 26.06.19) Centre, [email protected] Bihari Market, West of Jagdeo Path, Near Jagdeo Path Sheikhpra Flyover Approach, Rukanpura, PATNA – 800 014 (Bihar) 1840. Dr. Ekta Bhartiya 7033582754 CBWTF/1840/2019 M/S Chikitsa Clinic, Email: (Date: 26.06.19) XTTI, Ram Sagar Rai Market, Digha Ghat, [email protected] Digha, PATNA (Bihar) 1841. Zeba Alam 9431267445 CBWTF/1841/2019 M/S A Y Health Care, Email: (Date: 26.06.19) Tam Tam Padao, Near Phulwari PHC, [email protected] Phulwarisharif, PATNA – 801 505 (Bihar) 1842. Dr. Ayusha Kumari 7295038300 CBWTF/1842/2019 M/S Daant Dental Speciality Clinic, Email: (Date: 26.06.19) Below Raymond’s Showroom, Raj Plaza, [email protected] Anishabad, PATNA – 800 002 (Bihar) 1843. Dr. Tuhin Bharthuar 0612-2250286 CBWTF/1843/2019 M/S Bharthuar Dental Clinic, Road No. 39, 9631876448 (Date: 26.06.19) Gardanibagh, Email: PATNA – 800 002 (Bihar) [email protected] 1844. Ritesh Vatsa 7909055647 CBWTF/1844/2019 M/S Patna Dental Clinic And Implant Email: (Date: 26.06.19) Centre, [email protected] R. J. Complex, Makhania Kuan, PATNA – 800 004 (Bihar) 1845. Dr. Mukesh Kumar 9771424509 CBWTF/1845/2019 M/S K. -

Master Plan for Patna - 2031
IMPROVING DRAFT MASTER PLAN FOR PATNA - 2031 FINAL REPORT Prepared for, Department of Urban Development & Housing, Govt. of Bihar Prepared by, CEPT, Ahmadabad FINAL REPORT IMPROVING DRAFT MASTER PLAN FOR PATNA-2031 FINAL REPORT IMPROVING DRAFT MASTER PLAN FOR PATNA - 2031 Client: Urban Development & Housing Department Patna, Bihar i Prepared by: Center for Environmental Planning and Technology (CEPT) University Kasturbhai Lalbhai Campus, University Road, Navrangpura, Ahmedabad – 380 009 Gujarat State Tel: +91 79 2630 2470 / 2740 l Fax: +91 79 2630 2075 www.cept.ac.in I www.spcept.ac.in CEPT UNIVERSITY I AHMEDABAD i FINAL REPORT IMPROVING DRAFT MASTER PLAN FOR PATNA-2031 TABLE OF CONTENTS TABLE OF CONTENTS i LIST OF TABLES v LIST OF FIGURES vii LIST OF MAPS viii LIST of ANNEXURE ix 1 INTRODUCTION 10 1.1 Introduction 11 1.2 Planning Significance of Patna as a City 12 1.3 Economic Profile 14 1.4 Existing Land Use – Patna Municipal Corporation Area 14 1.5 Previous Planning Initiatives 16 1.5.1 Master Plan (1961-81) 16 1.5.2 Plan Update (1981-2001) 17 1.5.3 Master Plan 2001-21 18 1.6 Need for the Revision of the Master Plan 19 1.7 Methodology 20 1.7.1 Stage 1: Project initiation 20 1.7.2 Stage 02 and 03: Analysis of existing situation & Future projections and Concept Plan 21 1.7.3 Stage 04: Updated Base Map and Existing Land Use Map 21 1.7.4 Stage 5: Pre-final Master Plan and DCR 24 2 DELINEATION OF PATNA PLANNING AREA 25 i 2.1 Extent of Patna Planning Area (Project Area) 26 2.2 Delineation of Patna Planning Area (Project Area) 27 2.3 Delineated -
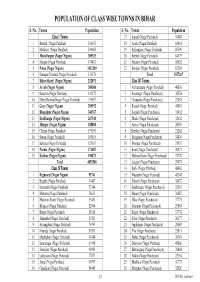
Population of Class Wise Towns in Bihar
POPULATION OF CLASS WISE TOWNS IN BIHAR S. No. Towns Population S. No. Towns Population Class I Towns 17 Supaul (Nagar Parishad) 54085 1 Bettiah (Nagar Parishad) 116670 18 Araria (Nagar Parishad) 60861 2 Motihari (Nagar Parishad) 100683 19 Kishanganj (Nagar Parishad) 85590 3 Muzaffarpur (Nagar Nigam) 305525 20 Beehat (Nagar Parishad) 64579 4 Hajipur (Nagar Parishad) 119412 21 Barauni (Nagar Parishad) 58628 5 Patna (Nagar Nigam) 1432209 22 Benipur (Nagar Parishad) 62203 6 Danapur Nizamat (Nagar Parishad) 131176 Total 1557267 7 Bihar Sharif (Nagar Nigam) 232071 Class III Towns 8 Arrah (Nagar Nigam) 203380 1 Narkatiaganj (Nagar Parishad) 40830 9 Sasaram (Nagar Parishad) 131172 2 Ramnagar (Nagar Panchayat) 38554 10 Dehri DalmiyaNagar (Nagar Parishad) 119057 3 Chanpatia (Nagar Panchayat) 22038 11 Gaya (Nagar Nigam) 389192 4 Raxaul (Nagar Parishad) 41610 12 Bhagalpur (Nagar Nigam) 340767 5 Sugauli (Nagar Panchayat) 31432 13 Darbhanga (Nagar Nigam) 267348 6 Dhaka (Nagar Panchayat) 32632 14 Munger (Nagar Nigam) 188050 7 Areraj (Nagar Panchayat) 20356 15 Chapra (Nagar Parishad) 179190 8 Sheohar (Nagar Panchayat) 21262 16 Siwan (Nagar Parishad) 109919 9 Bairgania (Nagar Panchayat) 34836 17 Saharsa (Nagar Parishad) 125167 10 Motipur (Nagar Panchayat) 21957 18 Purnia (Nagar Nigam) 171687 11 Kanti (Nagar Panchayat) 20871 19 Katihar (Nagar Nigam) 190873 12 Mahnar Bazar (Nagar Panchayat) 37370 Total 4853548 13 Lalganj (Nagar Panchayat) 29873 Class II Towns 14 Barh (Nagar Parishad) 48442 1 Begusarai (Nagar Nigam) 93741 15 Masaurhi (Nagar Parishad) 45248 -
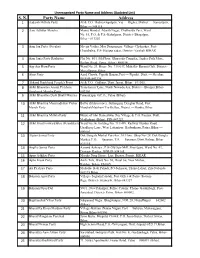
S. N. Party Name Address 1 Aadarsh Mithila Party at & P.O
Unrecognized Party Name and Address (Updated List) S. N. Party Name Address 1 Aadarsh Mithila Party At & P.O. Thahra Gopalpur, Via — Dighra, District — Samastipur, Bihar — 848115 2 Aam Adhikar Morcha Manoj Mandal, Adarsh Nagar, Chethariya Peer, Ward No. 15, P.O. & P.S.-Kahalgaon, District- Bhagalpur, Bihar - 813203 3 Aam Jan Party (Secular) Meena Vatika, Maa Durganagar, Village- Chaksakra, Post- Chandralya, P.S- Hajipur sadar, District- Vaishali BIHAR 4 Aam Janta Party Rashtriya Flat No. 804, 8th Floor, Gharouda Complex, Jagdeo Path More, Bailey. Road, Patna, Bihar - 800014. 5 Aap Aur Hum Party Ward No. 24, House No. 714/647, Mohalla- Basanti Gali, District- Muzaffarpur, Bihar 6 Aims Party Azad Chowk, Piprahi Bazaar,Post — Piprahi, Distt. — Sheohar, BIHAR-843334. 7 Akhand Jharkhand People's Front At & P.O.- Gidhaur, Distt. Jamui, Bihar - 811305. 8 Akhil Bharatiya Atyant Pichhara Transformer Lane, North Nawada,Ara, District - Bhojpur,Bihar- Sangharsh Morcha Party, 802301. 9 Akhil Bharatiya Desh Bhakt Morcha Puranderpur G.P.O., Patna (Bihar). 10 Akhil Bhartiya Manavadhikar Vichar Ballia (Jilebia more), Sultanganj, Deoghar Road, Post- Manch Party ManjhaliMatihani Via-Belhar, District — Banka, Bihar. 11 Akhil Bhartiya Mithila Party House of Shri Ratneshwar Jha, Village & P.O. Parjuar, Distt. Madhubani (Bihar), PIN-847229. 12 Akhil Hind Forward Bloc (Krantikari) Ward No.36, Holding No. 711/499, Railway Hunder Road, Upadhyay Lane, West Lohanipur, Kadamkuan, Patna, Bihar — 800003. 13 Alpjan Samaj Party Dak Bangala Market Parishar, 1st Floor, Shop No- 28, Dak Bangla Market, P.O. — Sasaram, P.S. — Sasaram, Distt. Rohtas, Bihar — 821115. 14 Angika Samaj Party Aanand Ashraya , P.0- Old Jute Mill, Sharifganj, Ward No. -

Sl. No. Centre Code Centre Name District Block Status Reasons 1 100935 PARAMPARA FOUNDATION Araria Araria Approved 2 98590 VIVEK
Sl. No. Centre Code Centre Name District Block Status Reasons 1 100935 PARAMPARA FOUNDATION Araria Araria Approved 2 98590 VIVEKANAND INSTITUTE OF INFORMATION TECHNOLOGY Araria Araria Approved 3 97500 DIVYA DRISTI BHARAT Araria Araria Approved 4 97613 SARTHAK FOUNDATION Araria Araria Approved 5 100610 AMBEDKAR SEVA SANSTHAN Araria Araria Approved 6 100101 YOUTH FORUM FORBEGANJ Araria Bhargama Approved 7 97688 LOK PRAGATI SEWA SANSTHAN Araria Kursakata Approved 8 98440 DIVYA DRISTI BHARAT PALASI Araria Palasi Approved 9 96053 SMB CLOUD INFOTECH Arwal Arwal Approved 10 96459 CICAT INSTITUE OF MANAGEMENT AND TECHNOLOGFY Arwal Arwal Approved 11 97015 PRABHAT WELFARE TRUST Arwal Arwal Approved 12 97434 MAX COMPUTER CENTRE Arwal Kaler Approved 13 96154 PERFECT DATATECH PRIVATE LIMITED Arwal Karpi Approved 14 101431 ADARSH KRITI FOUNDATION Arwal Karpi Approved 15 97792 KUSUM DEVI MAHILA SAMAJIK SEVA SANSTHAN Arwal Sonbhadra Banshi Approved Surypur 16 99176 COMPTECH TRAINING & TECH. PVT LTD Aurangabad Aurangabad Approved 17 97537 SAMAST MANAV JANKALYAN SANSTHAN Aurangabad Aurangabad Approved 18 95637 DATA PRO COMPUTERS PVT LTD Aurangabad Aurangabad Approved 19 95668 DATA PRO COMPUTERS PVT LTD Aurangabad Aurangabad Approved 20 96121 QUESS CORP LTD Aurangabad Aurangabad Approved 21 97428 PARMPARA FOUNDATION Aurangabad Aurangabad Approved 22 96169 MAHEYUGE EDUCATIONAL WELFARE SOC. Aurangabad Aurangabad Approved 23 99332 ZAKIRUDDIN GILANI MEMORIAL EDU. TRUST Aurangabad Aurangabad Approved 24 99334 ZAKIRUDDIN GILANI MEMORIAL EDU. TRUST Aurangabad Aurangabad Approved 25 100286 THITHOLI SAMAJIK EVAM SANSKRITIK DARPAN Aurangabad Aurangabad Approved 26 98128 Kriti Edify Pvt Ltd. Aurangabad Barun Approved 27 99872 NATIONAL RURAL DEVLOPMENT PROGRAM Aurangabad Barun Approved 28 101180 COMPTECH TRAINING & TECH. PVT LTD Aurangabad Daudnagar Approved 29 98262 Dharshan Institute of management & Pvt. -

List of Health Facilities Registered with I
List of Health facilities availing the services pertaining to transportation, treatment & disposal of Bio- Medical Waste generated by them at “Common Bio-Medical Waste Treatment Facility” installed at Indira Gandhi Institute of Medical Sciences, Sheikhpura, Patna. Sl. No. NAME OF HEALTH FACILITIES PATNA DISTRICT 1 J P HOSPITAL, AMBA PLAZA, BORING ROAD, PATNA JEEVAK SHALYA NILAYAN, W. ANANDPURI, BORING CANAL ROAD, PATNA - 2 800001 3 KANTH SURGICAL, BORING ROAD, PATNA 4 R S UROLOGY, WEST BORING CANALROAD, PATNA 5 LAXMI NURSHING HOMEWEST BORING CANAL ROAD, PATNA HARSH ADVANCED DIAGNOSTCS,BASEMENT, KUMAR TOWER,BORING CANAL 6 ROAD, PATNA 7 AMBIKA NURSING HOME ROAD NO. 12, S.K. NAGAR, PATNA 8 AROHI HOSPITAL 9 ASHIYANA NURSING HOME MAGISTRATE COLONY, SAGUNA MORE, PATNA ASHUTOSH MEMORIAL HOSPITAL,NEAR ADVANTAGE MEDIA, NEW BAILEY 10 ROAD (WEST OF CANAL), DANAPUR BAZAR PATNA, PATNA - 801503 11 ATLANTIS HOSPITAL, RAJA BAZAR,PATNA 12 DIVYA DRISHTI EYE CENTRE, RAJA BAZAR, PATNA 13 SEN DIAGNOSTIC, BUDH MARG, PATNA 14 DR. B. R. AMBEDKAR INSTITUTE OF DENTAL, NEW BAILLY ROAD, PATNA 15 GETWELL HOSPITAL RAJABAZAR, SHEIKHPURA, PATNA - 14 16 GOPAL NURSING HOME, BAILEY ROAD, PATNA 17 HI TECH HOSPITAL, SAGUNA MORE,PATNA 18 HMRI HOSPITALRAJA BAZAR, SHEKHPURA, PATNA-14 19 KESHAV HOSPITAL, SAGUNA MORE,DANAPUR, PATNA - 801503 20 MODERN SUPER SPECIALITYMANGAL MARKET, SHEIKHPURA PATNA - 14 21 POONAM SURGICAL & METARNITYCLINIC, SHEIKHPURA, BRAHM STHAN 22 RAMAWATI NURSING HOME, SHEIKHPURA, PATNA 23 S K JANCH GHAR SHIYANA ROAD, PATNA - 800014 24 SATYAM NURSING HOME BRAHM STHANI LANE, SHEIKHPURA, PATNA 25 SHYAMAL HOSPITAL MAURYA PATH, KHAJPURA, PATNA-14 26 SUN HOSPITAL, SHEIKH PURA, PATNA 27 THE SPECIALIST'S CLINIC, FRIENDS' COLONY, AG COLONY ROAD, PATNA 28 SHREE NURSHING HOME BIND TOLI, SHEIKHPURA, PATNA 29 ADVANCE NEURO DIAGNOSTIC 30 SANJEEVANI NEURO (CNS) S.K. -

Medical Waste Generated by Them at “Common Bio-Medical Waste Treatment Facility” Installed at Indira Gandhi Institute of Medical Sciences, Sheikhpura, Patna
List of Health facilities availing the services pertaining to transportation, treatment & disposal of Bio- Medical Waste generated by them at “Common Bio-Medical Waste Treatment Facility” installed at Indira Gandhi Institute of Medical Sciences, Sheikhpura, Patna. NEW REGISTRATION Registration of Firm’s After May – 2010 SI. Name & Address Contact No. Registration No. No. Allotted 1. Dr. Ajit Pradhan, 0612-2345895 CBWTF/01/2010 Jeevak Heart Hospital & Research 0612-2365814 Institute Pvt. Ltd., Fax: 0612-2363473 6-Doctor’s Colony, Kankarbagh, Patna E-mail: into@jeevak hospital.com 2. Dr. Kamal Kishore 9431422056 CBWTF/02/2010 Lab Pestisciences Intelligensio, E-mail: (Date: 14.06.2010 Vill.-Chakwara, P.o-Mazipur, Distt.- [email protected] Vaishali(Bihar) 3. Dr. Takeshwar Prasad Singh 0612-2302313 CBWTF/03/2010 Arvind Hospital Pvt. Ltd., 0612-6413672 (Date: 20.07.2010) Ashok Raj path, 0612-2300129 Patna-800004 E-mail: [email protected] 4. Dr. (Mrs.) V.Prasad, 0612-2253712 CBWTF/04/2010 Patna IV.F. @ Endo Surgery Centre, (Date: 20.07.2013) 20/B, Patliputra Colony, Patna-80000 5. Dr. (Mrs.) Kumud Kamini 0612-3362107 CBWTF/05/2010 M/S Hi-Tech Emergency Hospital,. Saguna More, Khagoul Road, Patna(Bihar) 6. Dr. Zaba Alam 9431267445 CBWTF/06/2010 M/S Alam Minorities Welfare Trust, (Date: 20.07.2013) Justica S.M.M. Alam Lane Near Mahavir Cancer Hosp. Pethiya Bazar, Phulwari Sharif Patna 7. Mr. Paras Nath Gupta, 0612-3265280 CBWTF/07/2010 M/S Modern Super Speciality (Date: 20.07.2013) Diagnostic & Research Centre, Mangal Market, Sheikhpura Patna-800014 8. Dr. Lal Path Labs Pvt. -

1) Network Hospital
1) Network Hospital Sr.No. Name of Hospital Address City State Pin Apollo Hospitals - New No.1, Old No.28, Platform 1 Sheshadripuram (U/o AHEL) Road, Near Mantri Mall, Bangalore Karnataka Sheshadripuram 560020 Aster CMI Hospital No. 43/2, NH-7, Hebbal, 2 Sahakara Nagar,, Bangalore Bangalore Karnataka Kodigehalli Gate, 560092 3 Bangalore Baptist Hospital Bellary Road,Hebbal., Bangalore Karnataka 560024 BGS Global Hospital BGS Health & Education City, 4 No # 67,Uttarahalli road, Bangalore Karnataka Kengeri, 560060 Columbia Asia - Sarjapura Survey No.46/2, Ward No.150,, 5 Bangalore Karnataka Road Ambalipura 560102 Columbia Asia Hospital - Sy No 10P & 12P 6 Whitefield Ramagondanhalli Village, Bangalore Karnataka Varthur Hobli Whitefield 560066 Columbia Asia Medical Kirloskar Buisiness Park Bellary 7 Bangalore Karnataka Centre Road, 560024 COLUMBIA ASIA REFERRAL 26/1,80 feet,Dr. Rajkumar 8 Bangalore Karnataka HOSPITAL- YESHWA Road, Malleshwaram(west) 560055 Fortis Hospitals Ltd - 9 154/9, Bannergatta, Bangalore Karnataka Bannerghatta Road 560076 Fortis Hospitals Ltd - 10 14, Cunningham Road, Bangalore Karnataka Cunningham Road 560052 Fortis Hospitals Ltd - 23 ,80feet Rd,Gurukripalaya 11 Nagarbhavi layout, opp to fab mall,2nd Bangalore Karnataka stage,Nagarbhavi 560072 12 Manipal Hospital 98, Rustom Bagh, Airport Road Bangalore Karnataka 560017 Manipal Hospitals #143, 212-215, EPIP Industrial 13 Whitefield Pvt. Ltd. (MHEPL) Area, Hoodi Village, KR Puram Bangalore Karnataka Hobli 560066 Narayana Hrudayalaya No. 258 / A, Bommasandra 14 Industrial Area, Hosur main Bangalore Karnataka road, Anekal Taluk 560099 People Tree Hospital(Unit of #2, Service Road, Tumkur Road, 15 TMI Health Care Pvt.) (NH-4), Opp.Hotel Vivanta, Bangalore Karnataka Yeshwantpur 560022 Sagar Hospital - Bangalore Shavige Malleshwara Hills, Behind DSI Campus, 16 Bangalore Karnataka Kumaraswamy Layout,Banashankari 560078 Sakra World Hospital No. -
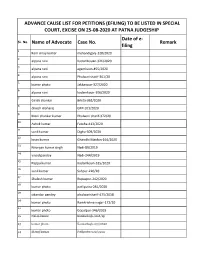
SL. No. Name of Advocate Case No. Date of E- Filing Remark ADVANCE
ADVANCE CAUSE LIST FOR PETITIONS (EFILING) TO BE LISTED IN SPECIAL COURT, EXCISE ON 25-08-2020 AT PATNA JUDGESHIP Date of e- SL. No. Name of Advocate Case No. Remark filing 1 Ram vinay kumar mehandiganj-128/2020 2 alpana rani Kadamkaukn-321/2020 3 alpana rani agamkaun-455/2020 4 alpana rani Phulwarisharif-361/20 5 kumar photo Jakkanpur-327/2020 6 alpana rani kadamkaun-306/2020 7 Girish shankar Bihita-362/2020 8 dinesh maharaj GRP-101/2020 9 Mani shankar kumar Phulwari sharif-372/20 10 Ashok kumar Fatuha-413/2020 11 sunil kumar Digha-309/2020 12 kiran kumar Ghandhi Maidan-164/2020 13 Niranjan kumar singh Nadi-89/2019 14 vinod pandey Nadi-244/2019 15 Pappu kumar Kadamkaun-325/2020 16 sunil kumar Sahpur-220/20 17 Shalesh kumar Rupaspur-242/2020 18 kumar photo patliputra-281/2020 19 sikandar pandey phulwarisharif-475/2018 20 kumar photo Ramkrishna nagar-172/20 21 kumar photo Gopalpur-346/2019 22 Nabin kumar Kankarbagh-1016/19 23 kumar photo Kankarbagh-231/2020 24 Manoj kumar Patliputra-220/2020 25 Pramod prasad Patliputra-222/2020 26 sunil kumar Pirbhore-202/2020 27 Harendra kumar Sahpur-199/2020 28 kumar photo Gaurichak-188/2020 29 Ram vinay kumar Dhanarua-231/2020 30 Randhir kumar Hawai adda-108/2020 31 kumar photo Barh-227/2020 32 Rohit kumar Barh-227/2020 33 Ajay kumar jakkanpur-364/2020 34 angesh rai Chowk-213/2020 35 Gyan prakash Punpun-77/2018 36 surendra prasad Phulwarisharif-281/2020 37 jacky sahni P.R.