Methionine Synthase Reductase Polymorphisms Are Associated with Serum Osteocalcin Levels in Postmenopausal Women
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Methionine Synthase Supports Tumor Tetrahydrofolate Pools
bioRxiv preprint doi: https://doi.org/10.1101/2020.09.05.284521; this version posted September 7, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. Methionine synthase supports tumor tetrahydrofolate pools Joshua Z. Wang1,2,#, Jonathan M. Ghergurovich1,3,#, Lifeng Yang1,2, and Joshua D. Rabinowitz1,2,* 1 Lewis-Sigler Institute for Integrative Genomics, Princeton University, Princeton, New Jersey, USA 2 Department of Chemistry, Princeton University, Princeton, New Jersey, USA 3 Department of Molecular Biology, Princeton University, Princeton, New Jersey, USA # These authors contributed equally to this work. *Corresponding author: Joshua Rabinowitz Department of Chemistry and the Lewis-Sigler Institute for Integrative Genomics, Princeton University, Washington Rd, Princeton, NJ 08544, USA Phone: (609) 258-8985; e-mail: [email protected] Conflict of Interest Disclosure: J.D.R. is a paid advisor and stockholder in Kadmon Pharmaceuticals, L.E.A.F. Pharmaceuticals, and Rafael Pharmaceuticals; a paid consultant of Pfizer; a founder, director, and stockholder of Farber Partners and Serien Therapeutics. JDR and JMG are inventors of patents in the area of folate metabolism held by Princeton University. 1 bioRxiv preprint doi: https://doi.org/10.1101/2020.09.05.284521; this version posted September 7, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. Abstract Mammalian cells require activated folates to generate nucleotides for growth and division. The most abundant circulating folate species is 5-methyl tetrahydrofolate (5-methyl- THF), which is used to synthesize methionine from homocysteine via the cobalamin-dependent enzyme methionine synthase (MTR). -

Defining Sources of Nutrient Limitation for Tumors By
Defining sources of nutrient limitation for tumors by Mark Robert Sullivan B.S. Molecular Biology and Chemistry University of Pittsburgh (2013) Submitted to the Department of Biology in Partial Fulfillment of the Requirements for the Degree of DOCTOR OF PHILOSOPHY at the MASSACHUSETTS INSTITUTE OF TECHNOLOGY June 2019 © 2019 Mark R. Sullivan. All rights reserved. The author hereby grants to MIT permission to reproduce and to distribute publicly paper and electronic copies of this thesis document in whole or in part in any medium now known or hereafter created. Signature of Author.......................................................................................................................... Department of Biology April 30, 2019 Certified by ...................................................................................................................................... Matthew G. Vander Heiden Associate Professor of Biology Thesis Supervisor Accepted by...................................................................................................................................... Amy E. Keating Professor of Biology Co-Director, Biology Graduate Committee 1 2 Defining sources of nutrient limitation for tumors by Mark Robert Sullivan Submitted to the Department of Biology on April 30, 2019 in Partial Fulfillment of the Requirements for the Degree of Doctor of Philosophy in Biology ABSTRACT Tumor growth requires that cancer cells accumulate sufficient biomass to grow and divide. To accomplish this, tumor cells must acquire -

Involvements of Hyperhomocysteinemia in Neurological Disorders
H OH metabolites OH Review Involvements of Hyperhomocysteinemia in Neurological Disorders Marika Cordaro 1,† , Rosalba Siracusa 2,† , Roberta Fusco 2 , Salvatore Cuzzocrea 2,3,* , Rosanna Di Paola 2,* and Daniela Impellizzeri 2 1 Department of Biomedical, Dental and Morphological and Functional Imaging, University of Messina, Via Consolare Valeria, 98125 Messina, Italy; [email protected] 2 Department of Chemical, Biological, Pharmaceutical and Environmental Sciences, University of Messina, 98166 Messina, Italy; [email protected] (R.S.); [email protected] (R.F.); [email protected] (D.I.) 3 Department of Pharmacological and Physiological Science, Saint Louis University School of Medicine, Saint Louis, MO 63104, USA * Correspondence: [email protected] (S.C.); [email protected] (R.D.P.); Tel.: +39-090-6765208 (S.C. & R.D.P.) † The authors equally contributed to the review. Abstract: Homocysteine (HCY), a physiological amino acid formed when proteins break down, leads to a pathological condition called hyperhomocysteinemia (HHCY), when it is over a definite limit. It is well known that an increase in HCY levels in blood, can contribute to arterial damage and several cardiovascular disease, but the knowledge about the relationship between HCY and brain disorders is very poor. Recent studies demonstrated that an alteration in HCY metabolism or a deficiency in folate or vitamin B12 can cause altered methylation and/or redox potentials, that leads to a modification on calcium influx in cells, or into an accumulation in amyloid and/or tau protein involving a cascade of events that culminate in apoptosis, and, in the worst conditions, neuronal death. The present review will thus summarize how much is known about the possible role of HHCY in neurodegenerative disease. -

MTHFR) and Methionine Synthase Reductase (MTRR
ANTICANCER RESEARCH 28 : 2807-2812 (2008) Methylenetetrahy drofolate Reductase (MTHFR) and Methionine Synthase Reductase (MTRR) Gene Polymorphisms as Risk Factors for Hepatocellular Carcinoma in a Korean Population SUN YOUNG KWAK 1,2 , UN KYUNG KIM 3, HYO JIN CHO 2, HEE KEUN LEE 3, HYE JIN KIM 2, NAM KEUN KIM 2 and SEONG GYU HWANG 1,2 1Department of Internal Medicine, 2Institute for Clinical Research, College of Medicine, Pochon CHA University, Seongnam; 3Department of Biology, College of Natural Sciences, Kyungpook National University, Daegu, South Korea Abstract. Hepatocellular carcinoma (HCC) is the third were increased risk factors for the disease. The MTHFR most frequent cause of cancer death in South Korea, but 1298A>C and the MTRR 66A>G genotypes were associated genetic susceptibility factors of HCC have not been examined with an increased risk of HCC in th is Korean population. extensively. Methylenetetrahydrofolate reductase (MTHFR) Further studies involving larger and varied populations and methionine synthase reductase (MTRR) play an essential could provide a potential tool for cancer risk assessment in role in both DNA synthesis and methylation and patients who are at risk of developing HCC. polymorphisms in the MTHFR gene, 677C>T, 1298A>C and the MTRR gene, 66A>G, are associated with several types of Hepatocellular carcinoma (HCC) is one of the most common malignancy. In this study, the allelic frequencies and malignant tumors with the third highest mortality rate among genotype distribution of three polymorphisms in the MTHFR malignancies in South Korea. According to a statistical report and MTRR genes from 96 hepatocellular carcinoma (HCC) in 2006, it occurred in 33.8/10,000 men and 11.2/10,000 patients and 201 controls were examined to assess the women in South Korea in 2005 (1). -

Recycling of Vitamin B12 and NAD+ Within the Pdu Microcompartment of Salmonella Enterica Shouqiang Cheng Iowa State University
Iowa State University Capstones, Theses and Graduate Theses and Dissertations Dissertations 2010 Recycling of vitamin B12 and NAD+ within the Pdu microcompartment of Salmonella enterica Shouqiang Cheng Iowa State University Follow this and additional works at: https://lib.dr.iastate.edu/etd Part of the Biochemistry, Biophysics, and Structural Biology Commons Recommended Citation Cheng, Shouqiang, "Recycling of vitamin B12 and NAD+ within the Pdu microcompartment of Salmonella enterica" (2010). Graduate Theses and Dissertations. 11713. https://lib.dr.iastate.edu/etd/11713 This Dissertation is brought to you for free and open access by the Iowa State University Capstones, Theses and Dissertations at Iowa State University Digital Repository. It has been accepted for inclusion in Graduate Theses and Dissertations by an authorized administrator of Iowa State University Digital Repository. For more information, please contact [email protected]. + Recycling of vitamin B12 and NAD within the Pdu microcompartment of Salmonella enterica by Shouqiang Cheng A dissertation submitted to the graduate faculty in partial fulfillment of the requirements for the degree of DOCTOR OF PHILOSOPHY Major: Biochemistry Program of Study Committee: Thomas A. Bobik, Major Professor Alan DiSpirito Basil Nikolau Reuben Peters Gregory J. Phillips Iowa State University Ames, Iowa 2010 Copyright © Shouqiang Cheng, 2010. All rights reserved. ii Table of contents Abstract............................................................................................................................. -

Supplementary Table S4. FGA Co-Expressed Gene List in LUAD
Supplementary Table S4. FGA co-expressed gene list in LUAD tumors Symbol R Locus Description FGG 0.919 4q28 fibrinogen gamma chain FGL1 0.635 8p22 fibrinogen-like 1 SLC7A2 0.536 8p22 solute carrier family 7 (cationic amino acid transporter, y+ system), member 2 DUSP4 0.521 8p12-p11 dual specificity phosphatase 4 HAL 0.51 12q22-q24.1histidine ammonia-lyase PDE4D 0.499 5q12 phosphodiesterase 4D, cAMP-specific FURIN 0.497 15q26.1 furin (paired basic amino acid cleaving enzyme) CPS1 0.49 2q35 carbamoyl-phosphate synthase 1, mitochondrial TESC 0.478 12q24.22 tescalcin INHA 0.465 2q35 inhibin, alpha S100P 0.461 4p16 S100 calcium binding protein P VPS37A 0.447 8p22 vacuolar protein sorting 37 homolog A (S. cerevisiae) SLC16A14 0.447 2q36.3 solute carrier family 16, member 14 PPARGC1A 0.443 4p15.1 peroxisome proliferator-activated receptor gamma, coactivator 1 alpha SIK1 0.435 21q22.3 salt-inducible kinase 1 IRS2 0.434 13q34 insulin receptor substrate 2 RND1 0.433 12q12 Rho family GTPase 1 HGD 0.433 3q13.33 homogentisate 1,2-dioxygenase PTP4A1 0.432 6q12 protein tyrosine phosphatase type IVA, member 1 C8orf4 0.428 8p11.2 chromosome 8 open reading frame 4 DDC 0.427 7p12.2 dopa decarboxylase (aromatic L-amino acid decarboxylase) TACC2 0.427 10q26 transforming, acidic coiled-coil containing protein 2 MUC13 0.422 3q21.2 mucin 13, cell surface associated C5 0.412 9q33-q34 complement component 5 NR4A2 0.412 2q22-q23 nuclear receptor subfamily 4, group A, member 2 EYS 0.411 6q12 eyes shut homolog (Drosophila) GPX2 0.406 14q24.1 glutathione peroxidase -
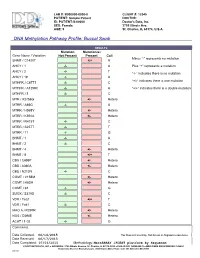
DNA Methylation Pathway Profile; Buccal Swab
LAB #: B000000-0000-0 CLIENT #: 12345 PATIENT: Sample Patient DOCTOR: ID: PATIENT-S-00000 Doctor's Data, Inc. SEX: Female 3755 Illinois Ave. AGE: 5 St. Charles, IL 60174, U.S.A. !DNA Methylation Pathway Profile; Buccal Swab RESULTS Mutation Mutation(s) Gene Name / Variation Not Present Present Call Minus “-“ represents no mutation SHMT / C1420T +/+ A AHCY / 1 -/- A Plus “+” represents a mutation AHCY / 2 -/- T “-/-“ indicates there is no mutation AHCY / 19 -/- A “+/-“ indicates there is one mutation MTHFR / C677T -/- C MTHFR / A1298C -/- A “+/+” indicates there is a double mutation MTHFR / 3 -/- C MTR / A2756G +/- Hetero MTRR / A66G -/- A MTRR / H595Y +/- Hetero MTRR / K350A +/- Hetero MTRR / R415T -/- C MTRR / S257T -/- T MTRR / 11 -/- G BHMT / 1 -/- A BHMT / 2 -/- C BHMT / 4 +/- Hetero BHMT / 8 +/+ T CBS / C699T +/- Hetero CBS / A360A +/- Hetero CBS / N212N -/- C COMT / V158M +/- Hetero COMT / H62H +/- Hetero COMT / 61 -/- G SUOX / S370S -/- C VDR / Taq1 +/+ T VDR / Fok1 -/- C MAO A / R297R +/- Hetero NOS / D298E +/- Hetero ACAT / 1-02 -/- G Comments: Date Collected: 06/14/2015 *For Research Use Only. Not for use in diagnostic procedures. Date Received: 06/17/2015 Date Completed: 07/03/2015 Methodology: MassARRAY iPLEXT platform by Sequenom ©DOCTOR’S DATA, INC. !!! ADDRESS: 3755 Illinois Avenue, St. Charles, IL 60174-2420 !!! CLIA ID NO: 14D0646470 !!! MEDICARE PROVIDER NO: 148453 Analyzed by Bioserve Biotechnologies, 9000 Virginia Manor Road, Suite 207, Beltsville, MD 20705 0001867 Methionine Metabolism Transmethylation & Transsulfuration Diagram Lab number: B000000-0000-0 DNA Methylatn BldSpt Page: 1 Patient: Sample Patient Client: 12345 Introduction Single nucleotide polymorphisms (SNPs) are DNA sequence variations, which may occur frequently in the population (at least one percent of the population.) They are different from disease mutations, which are very rare. -

DNA Methylation Pathway Profile; Blood Spot
LAB #: B000000-0000-0 CLIENT #: 12345 PATIENT: Sample Patient DOCTOR: ID: PATIENT-S-000000000 Doctor's Data, Inc. SEX: Male 3755 Illinois Ave AGE: 45 St. Charles, IL 60174 USA !DNA Methylation Pathway Profile; Blood Spot RESULTS Mutation Mutation(s) Gene Name / Variation Not Present Present Call SHMT / C1420T +/- Hetero Minus “-“ represents no mutation AHCY / 1 -/- A Plus “+” represents a mutation AHCY / 2 -/- T “-/-“ indicates there is no mutation AHCY / 19 -/- A MTHFR / C677T -/- C “+/-“ indicates there is one mutation MTHFR / A1298C -/- A “+/+” indicates there is a double mutation MTHFR / 3 -/- C MTR / A2756G +/- Hetero MTRR / A66G +/- Hetero MTRR / H595Y -/- C MTRR / K350A -/- A MTRR / R415T -/- C MTRR / S257T -/- T MTRR / 11 +/- Hetero BHMT / 1 -/- A BHMT / 2 +/- Hetero BHMT / 4 +/+ C BHMT / 8 +/+ T CBS / C699T -/- C CBS / A360A -/- C CBS / N212N -/- C COMT / V158M +/+ A COMT / H62H +/+ T COMT / 61 -/- G SUOX / S370S -/- CG VDR / Taq1 -/- C VDR / Fok1 -/- C MAO A / R297R -/- G NOS / D298E -/- G ACAT / 1-02 +/- Hetero Comments: Date Collected: 11/08/2012 *For Research Use Only. Not for use in diagnostic procedures. Date Received: 11/13/2012 Date Completed: 12/10/2012 Methodology: MassARRAY iPLEXT platform by Sequenom ©DOCTOR’S DATA, INC. !!! ADDRESS: 3755 Illinois Avenue, St. Charles, IL 60174-2420 !!! CLIA ID NO: 14D0646470 !!! MEDICARE PROVIDER NO: 148453 Analyzed by Bioserve Biotechnologies, 9000 Virginia Manor Road, Suite 207, Beltsville, MD 20705 0001867 Methionine Metabolism Transmethylation & Transsulfuration Diagram Lab number: B000000-0000-0 DNA Methylatn BldSpt Page: 1 Patient: Sample Patient Client: 12345 Introduction Single nucleotide polymorphisms (SNPs) are DNA sequence variations, which may occur frequently in the population (at least one percent of the population.) They are different from disease mutations, which are very rare. -

Polymorphisms in Methionine Synthase, Methionine Synthase Reductase and Serine Hydroxymethyltransferase, Folate and Alcohol Intake, and Colon Cancer Risk Susan E
University of South Carolina Scholar Commons Faculty Publications Epidemiology and Biostatistics 2008 Polymorphisms in Methionine Synthase, Methionine Synthase Reductase and Serine Hydroxymethyltransferase, Folate and Alcohol Intake, and Colon Cancer Risk Susan E. Steck University of South Carolina - Columbia, [email protected] Temitope O. Keku Lesley M. Butler Joseph Galanko Beri Massa See next page for additional authors Follow this and additional works at: https://scholarcommons.sc.edu/ sph_epidemiology_biostatistics_facpub Part of the Public Health Commons Publication Info Postprint version. Published in Journal of Nutrigenetics and Nutrigenomics, Volume 1, Issue 4, 2008, pages 196-204. Steck, S. E., Keku, T., Butler, L. M., Galanko, J., Massa, B., Millikan, R. C., & Sandler, R. S. (2008). Polymorphisms in methionine synthase, methionine synthase reductase and serine hydroxymethyltransferase, folate and alcohol intake, and colon cancer risk. Journal of Nutrigenetics and Nutrigenomics, 1(4), 196-204. DOI: 10.1159/000136651 © Journal of Nutrigenetics and Nutrigenomics, 2008, Karger http://content.karger.com/produktedb/produkte.asp?DOI=10.1159/000136651 This Article is brought to you by the Epidemiology and Biostatistics at Scholar Commons. It has been accepted for inclusion in Faculty Publications by an authorized administrator of Scholar Commons. For more information, please contact [email protected]. Author(s) Susan E. Steck, Temitope O. Keku, Lesley M. Butler, Joseph Galanko, Beri Massa, Robert C. Millikan, and Robert S. Sandler This article is available at Scholar Commons: https://scholarcommons.sc.edu/sph_epidemiology_biostatistics_facpub/303 NIH Public Access Author Manuscript J Nutrigenet Nutrigenomics. Author manuscript; available in PMC 2009 October 7. NIH-PA Author ManuscriptPublished NIH-PA Author Manuscript in final edited NIH-PA Author Manuscript form as: J Nutrigenet Nutrigenomics. -

Formate Metabolism in Health and Disease
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Enlighten Review Formate metabolism in health and disease Matthias Pietzke 1, Johannes Meiser 2, Alexei Vazquez 1,3,* ABSTRACT Background: Formate is a one-carbon molecule at the crossroad between cellular and whole body metabolism, between host and microbiome metabolism, and between nutrition and toxicology. This centrality confers formate with a key role in human physiology and disease that is currently unappreciated. Scope of review: Here we review the scientific literature on formate metabolism, highlighting cellular pathways, whole body metabolism, and interactions with the diet and the gut microbiome. We will discuss the relevance of formate metabolism in the context of embryonic development, cancer, obesity, immunometabolism, and neurodegeneration. Major conclusions: We will conclude with an outlook of some open questions bringing formate metabolism into the spotlight. Ó 2019 The Authors. Published by Elsevier GmbH. This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/). Keywords Formate metabolism; One-carbon-metabolism; Cancer; Immune system; Neurodegeneration; Obesity 1. INTRODUCTION product of anaerobic fermentation of some bacteria species popu- lating the gut microbiome [7]. The formate generated by the gut Formic acid (HCOOH) was first isolated from distillation of ant bodies, bacteria can enter the circulation, adding to the endogenous pool of and it was subsequently named using the Latin word for ant, formica formate or being used as substrate for the growth of other bacteria [1]. Ants and other insects accumulate formic acid in secretory with aerobic metabolism [8]. -

Genome, Proteome and Physiology of the Thermophilic Bacterium Anoxybacillus Flavithermus
Open Access Research2008SawetVolume al. 9, Issue 11, Article R161 Encapsulated in silica: genome, proteome and physiology of the thermophilic bacterium Anoxybacillus flavithermus WK1 Jimmy H Saw¤*‡‡, Bruce W Mountain¤†, Lu Feng¤‡§¶, Marina V Omelchenko¤¥, Shaobin Hou¤#, Jennifer A Saito*, Matthew B Stott†, Dan Li‡§¶, Guang Zhao‡§¶, Junli Wu‡§¶, Michael Y Galperin¥, Eugene V Koonin¥, Kira S Makarova¥, Yuri I Wolf¥, Daniel J Rigden**, Peter F Dunfield††, Lei Wang‡§¶ and Maqsudul Alam*# Addresses: *Department of Microbiology, University of Hawai'i, 2538 The Mall, Honolulu, HI 96822, USA. †GNS Science, Extremophile Research Group, 3352 Taupo, New Zealand. ‡TEDA School of Biological Sciences and Biotechnology, Nankai University, Tianjin 300457, PR China. §Tianjin Research Center for Functional Genomics and Biochip, Tianjin 300457, PR China. ¶Key Laboratory of Molecular Microbiology and Technology, Ministry of Education, Tianjin 300457, PR China. ¥National Center for Biotechnology Information, NLM, National Institutes of Health, Bethesda, MD 20894, USA. #Advance Studies in Genomics, Proteomics and Bioinformatics, College of Natural Sciences, University of Hawai'i, Honolulu, HI 96822, USA. **School of Biological Sciences, University of Liverpool, Crown Street, Liverpool L69 7ZB, UK. ††Department of Biological Sciences, University of Calgary, 2500 University Dr. NW, Calgary, Alberta T2N 1N4, Canada. ‡‡Current address: Bioscience Division, Los Alamos National Laboratory, Los Alamos, NM 87545, USA. ¤ These authors contributed equally to this work. Correspondence: Lei Wang. Email: [email protected]. Maqsudul Alam. Email: [email protected] Published: 17 November 2008 Received: 12 June 2008 Revised: 8 October 2008 Genome Biology 2008, 9:R161 (doi:10.1186/gb-2008-9-11-r161) Accepted: 17 November 2008 The electronic version of this article is the complete one and can be found online at http://genomebiology.com/2008/9/11/R161 © 2008 Saw et al.; licensee BioMed Central Ltd. -

Folate Deficiency Was Associated with Increased Alanine Aminotransferase and Glutamyl Transpeptidase Concentrations in a Chine
J Nutr Sci Vitaminol, 62, 265–271, 2016 Folate Deficiency Was Associated with Increased Alanine Aminotransferase and Glutamyl Transpeptidase Concentrations in a Chinese Hypertensive Population: A Cross-Sectional Study Wen-Xing LI1,2, Wei LI3, Jia-Qian CAO4,5, Haiyue YAN3, Yuanyuan SUN3, Hong ZHANG3, Qiang ZHANG3, Ling TANG3, Manman WANG3, Jing-Fei HUANG2,6,7 and Dahai LIU3,* 1 Institute of Health Sciences, Anhui University, Hefei 230601, Anhui, P. R. China 2 State Key Laboratory of Genetic Resources and Evolution, Kunming Institute of Zoology, Chinese Academy of Sciences, Kunming 650223, Yunnan, P. R. China 3 Center for Stem Cell and Translational Medicine, School of Life Sciences, Anhui University, Hefei 230601, Anhui, P. R. China 4 CAS Key Laboratory of Pathogenic Microbiology & Immunology, Institute of Microbiology, Chinese Academy of Sciences, Beijing 100101, P. R. China 5 University of Chinese Academy of Sciences, Beijing 100049, P. R. China 6 KIZ-SU Joint Laboratory of Animal Models and Drug Development, College of Pharmaceutical Sciences, Soochow University, Suzhou 215123, Jiangsu, P. R. China 7 Collaborative Innovation Center for Natural Products and Biological Drugs of Yunnan, Kunming 650223, Yunnan, P. R. China (Received February 29, 2016) Summary Alanine aminotransferase (ALT), aspartate transaminase (AST), and glutamyl transpeptidase (GGT) were three key enzymes in the hepatic metabolism. This study aimed to investigate the effect of homocysteine (Hcy) metabolism gene polymorphisms and serum Hcy and folate level on the hepatic functions in a Chinese hypertensive population. A repre- sentative sample with 480 subjects aged 28–75 was enrolled in 2005.9–2005.12 from six hospitals in different Chinese regions.