WO 2008/025673 Al
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Polycyclic Aromatic Hydrocarbon Structure Index
NIST Special Publication 922 Polycyclic Aromatic Hydrocarbon Structure Index Lane C. Sander and Stephen A. Wise Chemical Science and Technology Laboratory National Institute of Standards and Technology Gaithersburg, MD 20899-0001 December 1997 revised August 2020 U.S. Department of Commerce William M. Daley, Secretary Technology Administration Gary R. Bachula, Acting Under Secretary for Technology National Institute of Standards and Technology Raymond G. Kammer, Director Polycyclic Aromatic Hydrocarbon Structure Index Lane C. Sander and Stephen A. Wise Chemical Science and Technology Laboratory National Institute of Standards and Technology Gaithersburg, MD 20899 This tabulation is presented as an aid in the identification of the chemical structures of polycyclic aromatic hydrocarbons (PAHs). The Structure Index consists of two parts: (1) a cross index of named PAHs listed in alphabetical order, and (2) chemical structures including ring numbering, name(s), Chemical Abstract Service (CAS) Registry numbers, chemical formulas, molecular weights, and length-to-breadth ratios (L/B) and shape descriptors of PAHs listed in order of increasing molecular weight. Where possible, synonyms (including those employing alternate and/or obsolete naming conventions) have been included. Synonyms used in the Structure Index were compiled from a variety of sources including “Polynuclear Aromatic Hydrocarbons Nomenclature Guide,” by Loening, et al. [1], “Analytical Chemistry of Polycyclic Aromatic Compounds,” by Lee et al. [2], “Calculated Molecular Properties of Polycyclic Aromatic Hydrocarbons,” by Hites and Simonsick [3], “Handbook of Polycyclic Hydrocarbons,” by J. R. Dias [4], “The Ring Index,” by Patterson and Capell [5], “CAS 12th Collective Index,” [6] and “Aldrich Structure Index” [7]. In this publication the IUPAC preferred name is shown in large or bold type. -

ALABAMA SEAFOOD SURVEILLANCE SAMPLES NPH = Naphthalene, FLU = Fluorene, PHN = Phenanthrene, ANT = Anthracene, FLA = Fluoranthene
ALABAMA SEAFOOD SURVEILLANCE SAMPLES NPH = Naphthalene, FLU = Fluorene, PHN = Phenanthrene, ANT = Anthracene, FLA = Fluoranthene, Polycyclic Aromatic Hydrocarbon (PAH) and PYR = Pyrene, BaA = Benz(a)anthracene, CHR = Chrysene, BbF = Benzo(b)fluoranthene, DOSS Results Summary BkF = Benzo(k)fluoranthene, BaP = Benzo(a)pyrene, DBA = Dibenz(a,h)anthracene, IcdPy = Indeno(1,2,3-cd)pyrene, DOSS = Dioctylsulfosuccinate **The estimated maximum total PAH value represents a "worst case" estimate of the PAHs including alkyl homologs that could potentially be in the that happens to yield fluorescence responsesample. Results reported using FDACS Screening Method 521, based on It may include fluorescent compounds other than PAHs and background signal that happens to yield fluorescence response FDA LC Fluorescence Screening Method and FDACS DOSS Levels of Concern bases on FDA's Protocol for Interpretation and Use of Sensory Testing and Analytical Chemistry Results for Reopening In order to "PASS" Method 522 based on FDA's Determination of Levels of Concern (ppm) Oil-Impacted Areas closed to Seafood Harvesting. 7/26/10 samples must not Dioctylsulfosuccinate in Select Seafoods using LC/MS Shrimp and Crab 123 246 1846 246 185 1.32 1.32 13.2 0.132 0.132 1.32 61.5 500 exceed any Sorted by seafood type (crab, finfish, oyster, shrimp), Oysters 133 267 2000 267 200 1.43 143 1.43 14.3 0.143 0.143 1.43 66.5 500 FDA Levels of harvest area and sample # Finfish 32.7 65.3 490 65.3 49 0.35 35 0.35 0.35 0.035 0.035 0.35 16.35 100 Concern <LOD = less than Limit of Detection, -

Azulene—A Bright Core for Sensing and Imaging
molecules Review Azulene—A Bright Core for Sensing and Imaging Lloyd C. Murfin * and Simon E. Lewis Department of Chemistry, University of Bath, Bath BA2 7AY, UK; [email protected] * Correspondence: lloyd.murfi[email protected] Abstract: Azulene is a hydrocarbon isomer of naphthalene known for its unusual colour and fluores- cence properties. Through the harnessing of these properties, the literature has been enriched with a series of chemical sensors and dosimeters with distinct colorimetric and fluorescence responses. This review focuses specifically on the latter of these phenomena. The review is subdivided into two sec- tions. Section one discusses turn-on fluorescent sensors employing azulene, for which the literature is dominated by examples of the unusual phenomenon of azulene protonation-dependent fluorescence. Section two focuses on fluorescent azulenes that have been used in the context of biological sensing and imaging. To aid the reader, the azulene skeleton is highlighted in blue in each compound. Keywords: fluorescence; azulene; sensor; dosimeter; bioimaging; chemosensor; chemodosimeter 1. Introduction Azulene, 1, is an isomer of naphthalene, 2, composed of fused 5- and 7-membered ring systems (Figure1) and named for its vibrant blue colour. Unlike naphthalene, azulene is a non-alternant hydrocarbon, possessing nodal points at C-2 and C-6 of the HOMO and C-1 and C-3 of the LUMO [1]. The location of these nodes results in low electronic repulsion in the S1 singlet excited state, affording a relatively small HOMO-LUMO gap. Hence, the S0!S1 transition arises from absorption in the visible region. Conversely, in naphthalene, coefficient magnitudes remain consistent for each position in both the HOMO Citation: Murfin, L.C.; Lewis, S.E. -

Polycyclic Aromatic Hydrocarbons (Pahs)
Polycyclic Aromatic Hydrocarbons (PAHs) Factsheet 4th edition Donata Lerda JRC 66955 - 2011 The mission of the JRC-IRMM is to promote a common and reliable European measurement system in support of EU policies. European Commission Joint Research Centre Institute for Reference Materials and Measurements Contact information Address: Retiewseweg 111, 2440 Geel, Belgium E-mail: [email protected] Tel.: +32 (0)14 571 826 Fax: +32 (0)14 571 783 http://irmm.jrc.ec.europa.eu/ http://www.jrc.ec.europa.eu/ Legal Notice Neither the European Commission nor any person acting on behalf of the Commission is responsible for the use which might be made of this publication. Europe Direct is a service to help you find answers to your questions about the European Union Freephone number (*): 00 800 6 7 8 9 10 11 (*) Certain mobile telephone operators do not allow access to 00 800 numbers or these calls may be billed. A great deal of additional information on the European Union is available on the Internet. It can be accessed through the Europa server http://europa.eu/ JRC 66955 © European Union, 2011 Reproduction is authorised provided the source is acknowledged Printed in Belgium Table of contents Chemical structure of PAHs................................................................................................................................. 1 PAHs included in EU legislation.......................................................................................................................... 6 Toxicity of PAHs included in EPA and EU -

Design and Synthesis of Novel Symmetric Fluorene-2,7-Diamine Derivatives As Potent Hepatitis C Virus Inhibitors
pharmaceuticals Article Design and Synthesis of Novel Symmetric Fluorene-2,7-Diamine Derivatives as Potent Hepatitis C Virus Inhibitors Mai H. A. Mousa 1, Nermin S. Ahmed 1,*, Kai Schwedtmann 2, Efseveia Frakolaki 3, Niki Vassilaki 3, Grigoris Zoidis 4 , Jan J. Weigand 2 and Ashraf H. Abadi 1,* 1 Department of Pharmaceutical Chemistry, Faculty of Pharmacy and Biotechnology, German University in Cairo, Cairo 11835, Egypt; [email protected] 2 Faculty of Chemistry and Food Chemistry, Technische Universität Dresden, 01062 Dresden, Germany; [email protected] (K.S.); [email protected] (J.J.W.) 3 Molecular Virology Laboratory, Hellenic Pasteur Institute, 11521 Athens, Greece; [email protected] (E.F.); [email protected] (N.V.) 4 Department of Pharmacy, Division of Pharmaceutical Chemistry, School of Health Sciences, National and Kapodistrian University of Athens, 15771 Athens, Greece; [email protected] * Correspondence: [email protected] (N.S.A.); [email protected] (A.H.A.); Tel.: +202-27590700 (ext. 3429) (N.S.A.); +202-27590700 (ext. 3400) (A.H.A.); Fax: +202-27581041 (N.S.A. & A.H.A.) Abstract: Hepatitis C virus (HCV) is an international challenge. Since the discovery of NS5A direct-acting antivirals, researchers turned their attention to pursue novel NS5A inhibitors with optimized design and structure. Herein we explore highly potent hepatitis C virus (HCV) NS5A Citation: Mousa, M.H.A.; Ahmed, inhibitors; the novel analogs share a common symmetrical prolinamide 2,7-diaminofluorene scaffold. N.S.; Schwedtmann, K.; Frakolaki, E.; Modification of the 2,7-diaminofluorene backbone included the use of (S)-prolinamide or its isostere Vassilaki, N.; Zoidis, G.; Weigand, J.J.; (S,R)-piperidine-3-caboxamide, both bearing different amino acid residues with terminal carbamate Abadi, A.H. -

Technical Background Document (U.S
Part 5: CHEMICAL-SPECIFIC PARAMETERS Chemical-specific parameters required for calculating soil screening levels include the organic carbon normalized soil-water partition coefficient for organic compounds (Koc), the soil-water partition coefficient for inorganic constituents (Kd), water solubility (S), Henry's law constant (HLC, HN), air diffusivity (Di,a), and water diffusivity (Di,w). In addition, the octanol-water partition coefficient (Kow) is needed to calculate Koc values. This part of the background document describes the collection and compilation of these parameters for the SSL chemicals. With the exception of values for air diffusivity (Di,a), water diffusivity (Di,w), and certain Koc values, all of the values used in the development of SSLs can be found in the Superfund Chemical Data Matrix (SCDM). SCDM is a computer code that includes more than 25 datafiles containing specific chemical parameters used to calculate factor and benchmark values for the Hazard Ranking System (HRS). Because SCDM datafiles are regularly updated, the user should consult the most recent version of SCDM to ensure that the values are up to date. 5.1 Solubility, Henry's Law Constant, and Kow Chemical-specific values for solubility, Henry's law constant (HLC), and Kow were obtained from SCDM. In the selection of the value for SCDM, measured or analytical values are favored over calculated values. However, in the event that a measured value is not available, calculated values are used. Table 36 presents the solubility, Henry's law constant, and Kow values taken from SCDM and used to calculate SSLs. Henry's law constant values were available for all but two of the constituents of interest. -

Substituent Effects in Hydrogen Abstraction by Trichloromethyl Radical from 10-Substituted-9
AN ABSTRACT OF THE THESIS OF GARY SCOTT NOLAN for the degree of MASTER OF SCIENCE in CHEMISTRY presented on e IP tcv-vi Title: SUBSTITUENT EFFECTS IN HYDROGEN ABSTRACTION BY TRICHLOROMETHYL RADICAL FROM 10-SUBSTITUTED-9- METHYLANTHRACENES; A LINEAR FREE ENERGY STUDY Abstract approved: Redacted for Privacy Dr. Gerald° Jay Gleicher Hydrogen abstraction by the trichloromethy-1 radical from a series of 10-substituted-9-methylanthracenes at 70° has been examined. The logarithms of the relative rates, measured against hydrogen abstraction from fluorene, correlate very well with sigma plus parameters within the Hammett formalism. A rho value of -0.78 ± 0.05 was observed with a correlation constant of.99 and a standard regression from the mean of .06.This result implies a charge separated character to the transition state with stabilization by electron donating groups. The present work serves as a rigorous test of "Pryor's Postulate".That statement holds that the reaction con- stant or rho value for aryl methyl hydrogen abstraction is, within experimental uncertainties, the same as that for aromatic substitu- tion into the nonmethylated arene.Since a substitution study within the Hammett framework has already been performed, the present study makes a comparison of rho values possible.The rho value obtained in the trichloromethylation of 9-substituted-anthracenes at 70° was -0.83 ± 0.04.This agrees well with the present result. This agreement tends to substantiate and extend "Pryor's Postulate" by treating relatively selective radicals and polycyclic -

Anlage II: Forschungsprofile Der Antragstellenden Wissenschaftler
Inhaltsverzeichnis 1 Anlage II: Forschungsprofile der antragstellenden Wissenschaftler Inhaltsverzeichnis: Prof. Dr. Matthias BELLER (A1) ............................................................................................2 Prof. Dr. Uwe ROSENTHAL (A2) Sprecher des Graduiertenkollegs 1213..................................................................................19 Priv.-Doz. Dr. Detlef HELLER (A3) ......................................................................................26 Prof. Dr. Armin BÖRNER (B1) .............................................................................................30 Priv.-Doz. Dr. Sergey VEREVKIN (B2) ................................................................................37 Prof. Dr. Udo KRAGL (B3) ...................................................................................................44 Prof. Dr. Peter LANGER (B4) ...............................................................................................49 Prof. Dr.-Ing. habil. Kerstin THUROW (C1).........................................................................66 Prof. Dr.-Ing. Norbert STOLL (C2).......................................................................................72 Priv.-Doz. Dr. Angelika BRÜCKNER (C3) ...........................................................................76 Fortsetzungsantrag GRK 1213, Anlage II 2 Forschungsprofil Matthias Beller Prof. Dr. Matthias BELLER (A1) Organische Chemie Leibniz-Institut für Katalyse an der Universität Rostock e.V. (LIKAT) A.-Einstein-Str. -

Screening and Determination of Polycyclic Aromatic Hydrocarbons
METHOD NUMBER: C-002.01 POSTING DATE: November 1, 2017 POSTING EXPIRATION DATE: November 1, 2023 PROGRAM AREA: Seafood METHOD TITLE: Screening and Determination of Polycyclic Aromatic Hydrocarbons in Seafoods Using QuEChERS-Based Extraction and High-Performance Liquid Chromatography with Fluorescence Detection VALIDATION STATUS: Equivalent to Level 3 Multi-laboratory validation (MLV) AUTHOR(S): Samuel Gratz, Angela Mohrhaus Bryan Gamble, Jill Gracie, David Jackson, John Roetting, Laura Ciolino, Heather McCauley, Gerry Schneider, David Crockett, Douglas Heitkemper, and Fred Fricke (FDA Forensic Chemistry Center) METHOD SUMMARY/SCOPE: Analyte(s): Polycyclic aromatic hydrocarbons (PAH): acenaphthene, anthracene, benzo[a]anthracene, benzo[a]pyrene, benzo[b]fluoranthene, benzo[g,h,i]perylene, benzo[k]fluoranthene, chrysene, dibenzo[a,h]anthracene, fluoranthene, fluorene, indeno[1,2,3-cd]pyrene, naphthalene, phenanthrene, pyrene Matrices: Oysters, shrimp, crabs, and finfish The method provides a procedure to screen for fifteen targeted parent polycyclic aromatic hydrocarbons (PAHs) and provides an estimate of total PAH concentration including alkylated homologs in oysters, shrimp, crabs, and finfish. PAHs are extracted from seafood matrices using a modified QuEChERS sample preparation procedure. The method utilizes High-Performance Liquid Chromatography with Fluorescence Detection (HPLC-FLD) for the determination step. This procedure is applicable to screen a variety of seafood matrices including oysters, shrimp, finfish and crab for the presence of parent PAHs and the common alkylated homologs due to oil contamination. This method was originally developed and validated in response to the 2010 Gulf of Mexico oil spill. REVISION HISTORY: OTHER NOTES: Method was originally posted on November 1, 2017. It was approved for re-posting by the Chemistry Research Coordination Group for 3 years in December 2020. -
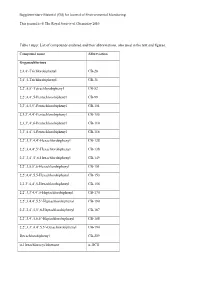
List of Compounds Analysed and Their Abbreviations, Also Used in the Text and Figures
Supplementary Material (ESI) for Journal of Environmental Monitoring This journal is © The Royal Society of Chemistry 2010 Table 1supp: List of compounds analysed and their abbreviations, also used in the text and figures. Compound name Abbreviation Organochlorines 2,4,4’-Trichlorobiphenyl CB-28 2,4’,5-Trichlorobiphenyl CB-31 2,2’,5,5’-Tetrachlorobiphenyl CB-52 2,2’,4,4’,5-Pentachlorobiphenyl CB-99 2,2’,4,5,5’-Pentachlorobiphenyl CB-101 2,3,3’,4,4’-Pentachlorobiphenyl CB-105 2,3,3’,4’,6-Pentachlorobiphenyl CB-110 2,3’,4,4’,5-Pentachlorobiphenyl CB-118 2,2’,3,3’,4,4’-Hexachlorobiphenyl CB-128 2,2’,3,4,4’,5’-Hexachlorobiphenyl CB-138 2,2’,3,4’,5’,6-Hexachlorobiphenyl CB-149 2,2’,3,5,5’,6-Hexachlorobiphenyl CB-151 2,2’,4,4’,5,5-Hexachlorobiphenyl CB-153 2,3,3’,4,4’,5-Hexachlorobiphenyl CB-156 2,2’,3,3’4,4’,5-Heptachlorobiphenyl CB-170 2,2’,3,4,4’,5,5’-Heptachlorobiphenyl CB-180 2,2’,3,4’,5,5’,6-Heptachlorobiphenyl CB-187 2,2’,3,4’,5,6,6’-Heptachlorobiphenyl CB-188 2,2’,3,3’,4,4’,5,5’-Octachlorobiphenyl CB-194 Decachlorobiphenyl CB-209 α-Hexachlorocyclohexane α-HCH Supplementary Material (ESI) for Journal of Environmental Monitoring This journal is © The Royal Society of Chemistry 2010 β-Hexachlorocyclohexane β-HCH γ-Hexachlorocyclohexane γ-HCH Hexachlorobenzene HCB Trans-nonachlor TNC p,p’-dichloro-diphenyl-trichloroethane p,p’-DDT p,p’-dichloro-diphenyl-dichloroethylene p,p’-DDE p,p’-dichloro-diphenyl-dichloroethane p,p’-DDD o,p’-dichloro-diphenyl-trichloroethane o,p’-DDT o,p’-dichloro-diphenyl-dichloroethylene o,p’-DDE PAHs Naphthalene -

Study of the Water Quality Index and Polycyclic Aromatic Hydrocarbon for a River Receiving Treated Landfill Leachate
water Article Study of the Water Quality Index and Polycyclic Aromatic Hydrocarbon for a River Receiving Treated Landfill Leachate Brenda Tan Pei Jian 1, Muhammad Raza Ul Mustafa 1,* , Mohamed Hasnain Isa 2, Asim Yaqub 3 and Ho Yeek Chia 1 1 Department of Civil and Environmental Engineering, Universiti Teknologi PETRONAS, Seri Iskandar 32610, Perak Darul Ridzuan, Malaysia; [email protected] (B.T.P.J.); [email protected] (H.Y.C.) 2 Civil Engineering Programme, Faculty of Engineering, Universiti Teknologi Brunei, Tungku Highway, Gadong BE1410, Brunei; [email protected] 3 Department of Environmental Sciences, COMSATS University Islamabad Abbottabad Campus, Abbottabad 22060, Pakistan; [email protected] * Correspondence: [email protected] Received: 29 July 2020; Accepted: 18 September 2020; Published: 16 October 2020 Abstract: Rising solid waste production has caused high levels of environmental pollution. Population growth, economic patterns, and lifestyle patterns are major factors that have led to the alarming rate of solid waste production. Generally, solid wastes such as paper, wood, and plastic are disposed into landfills due to its low operation and maintenance costs. However, leachate discharged from landfills could be a problem in surfaces and groundwater if not adequately treated. This study investigated the patterns of the water quality index (WQI) and polycyclic aromatic hydrocarbons (PAH) along Johan River in Perak, Malaysia, which received treated leachate from a nearby landfill. An artificial neural network (ANN) was also applied to predict WQI and PAH concentration of the river. Seven sampling stations were chosen along the river. The stations represented the upstream of leachate discharge, point of leachate discharge, and five locations downstream of the landfill. -

Pka Values of N-Aryl Imidazolinium Salts, Their Higher Homologues, and Formamidinium Salts in Dimethyl Sulfoxide” Nicholas Konstandaras, Michelle H
Electronic Supplementary Material (ESI) for Organic & Biomolecular Chemistry. This journal is © The Royal Society of Chemistry 2020 Electronic supporting information for “The pKa values of N-aryl imidazolinium salts, their higher homologues, and formamidinium salts in dimethyl sulfoxide” Nicholas Konstandaras, Michelle H. Dunn, Ena T. Luis, Marcus L. Cole and Jason B. Harper School of Chemistry, University of New South Wales, Sydney, NSW, 2052, Australia. Synthesis of the fluorenes 1, 2, 3, 5 and 7 2 Absorbance data for the deprotonated form of each of the indicators 1-7 7 Synthesis of the salts 8-11 and pKa data 8 Synthesis of the salts 12-14 and pKa data 12 Synthesis of the salt 15 and pKa data 15 pKa Data for salt 16 17 NMR experiments to determine the relative acidity of salts 10 and 15 18 Identifying conformers in NMR spectra of the formamidinium salts 19-22 21 Determination of pKa values for formadinium salts 19-22 23 Synthesis of the formamidinium salts 19-22 and pKa data 26 NMR spectra of all prepared salts 34 References 44 Synthesis of the fluorenes 1, 2, 3, 5 and 7 9-Isopropylsulfane-9H-fluorene 11 In a method analogous to literature,1 a solution of aqueous sodium hydroxide (0.57 g, 14 mmol, 14 M) was added in one portion to a solution of propanethiol (0.49 g 6.4 mmol) in THF (6 mL) under nitrogen. A solution of 9-bromo-9H-fluorene (1.56 g, 6.36 mmol) in THF (2 mL) was then added in one portion.