Annals of Behavioral Neuroscience Symmetrical Central Tegmental Tract Hyperintensity on T2-Weighted Images in Pediatrics: a Systematic Review
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Distance Learning Program Anatomy of the Human Brain/Sheep Brain Dissection
Distance Learning Program Anatomy of the Human Brain/Sheep Brain Dissection This guide is for middle and high school students participating in AIMS Anatomy of the Human Brain and Sheep Brain Dissections. Programs will be presented by an AIMS Anatomy Specialist. In this activity students will become more familiar with the anatomical structures of the human brain by observing, studying, and examining human specimens. The primary focus is on the anatomy, function, and pathology. Those students participating in Sheep Brain Dissections will have the opportunity to dissect and compare anatomical structures. At the end of this document, you will find anatomical diagrams, vocabulary review, and pre/post tests for your students. The following topics will be covered: 1. The neurons and supporting cells of the nervous system 2. Organization of the nervous system (the central and peripheral nervous systems) 4. Protective coverings of the brain 5. Brain Anatomy, including cerebral hemispheres, cerebellum and brain stem 6. Spinal Cord Anatomy 7. Cranial and spinal nerves Objectives: The student will be able to: 1. Define the selected terms associated with the human brain and spinal cord; 2. Identify the protective structures of the brain; 3. Identify the four lobes of the brain; 4. Explain the correlation between brain surface area, structure and brain function. 5. Discuss common neurological disorders and treatments. 6. Describe the effects of drug and alcohol on the brain. 7. Correctly label a diagram of the human brain National Science Education -

Magnetic Resonance Imaging of Multiple Sclerosis: a Study of Pulse-Technique Efficacy
691 Magnetic Resonance Imaging of Multiple Sclerosis: A Study of Pulse-Technique Efficacy Val M. Runge1 Forty-two patients with the clinical diagnosis of multiple sclerosis were examined by Ann C. Price1 proton magnetic resonance imaging (MRI) at 0.5 T. An extensive protocol was used to Howard S. Kirshner2 facilitate a comparison of the efficacy of different pulse techniques. Results were also Joseph H. Allen 1 compared in 39 cases with high-resolution x-ray computed tomography (CT). MRI revealed characteristic abnormalities in each case, whereas CT was positive in only 15 C. Leon Partain 1 of 33 patients. Milder grades 1 and 2 disease were usually undetected by CT, and in all A. Everette James, Jr.1 cases, the abnormalities noted on MRI were much more extensive than on CT. Cerebral abnormalities were best shown with the T2-weighted spin-echo sequence (TE/TR = 120/1000); brainstem lesions were best defined on the inversion-recovery sequence (TE/TI/TR =30/400/1250). Increasing TE to 120 msec and TR to 2000 msec heightened the contrast between normal and abnormal white matter. However, the signal intensity of cerebrospinal fluid with this pulse technique obscured some abnormalities. The diagnosis of multiple sclerosis continues to be a clinical challenge [1,2). The lack of an objective means of assessment further complicates the evaluation of treatment regimens. Evoked potentials, cerebrospinal fluid (CSF) analysis , and computed tomography (CT) are currently used for diagnosis, but all lack sensitivity and/or specificity. Furthermore, postmortem examinations demonstrate many more lesions than those suggested by clinical means [3). -

Brainstem: Structure & Its Mode of Action
Journal of Neurology & Neurophysiology 2021, Vol.12, Issue 3, 521 Opinion Brainstem: Structure & Its Mode of action Karthikeyan Rupani Research Fellow, Tata Medical Centre, India. Corresponding Author* The brainstem is exceptionally little, making up around as it were 2.6 percent of the brain's add up to weight. It has the basic parts of directing cardiac, and Rupani K, respiratory work, making a difference to control heart rate and breathing rate. Research Fellow, Tata Medical Centre, India; It moreover gives the most engine and tactile nerve supply to the confront and E-mail: [email protected] neck by means of the cranial nerves. Ten sets of cranial nerves come from the brainstem. Other parts incorporate the direction of the central apprehensive Copyright: 2021 Rupani K. This is an open-access article distributed under the framework and the body's sleep cycle. It is additionally of prime significance terms of the Creative Commons Attribution License, which permits unrestricted within the movement of engine and tangible pathways from the rest of the use, distribution, and reproduction in any medium, provided the original author brain to the body, and from the body back to the brain. These pathways and source are credited. incorporate the corticospinal tract (engine work), the dorsal column-medial lemniscus pathway and the spinothalamic tract [3]. The primary part of the brainstem we'll consider is the midbrain. The midbrain Received 01 March 2021; Accepted 15 March 2021; Published 22 March 2021 (too known as the mesencephalon) is the foremost prevalent of the three districts of the brainstem. It acts as a conduit between the forebrain over and the pons and cerebellum underneath. -

Isolated Medulla Oblongata Function After Severe Traumatic Brain Injury
J Neurol Neurosurg Psychiatry 2001;70:127–129 127 J Neurol Neurosurg Psychiatry: first published as 10.1136/jnnp.70.1.127 on 1 January 2001. Downloaded from SHORT REPORT Isolated medulla oblongata function after severe traumatic brain injury E F M Wijdicks, J L D Atkinson, H Okazaki Abstract reflexes were absent. Brain CT documented a The objective was to report the first large left epidural supratentorial haematoma in pathologically confirmed case of partly addition to subarachnoid blood in the basal functionally preserved medulla oblongata cisterns and fourth ventricle and intraparen- in a patient with catastrophic traumatic chymal haemorrhage in both cerebellar pedun- brain injury. cles and pons. Laboratory tests including A patient is described with epidural serum alcohol concentration and urinary toxi- haematoma with normal breathing and cology screen were unremarkable. He was blood pressure and a retained coughing taken to surgery as an emergency for left crani- reflex brought on only by catheter suc- otomy and removal of epidural haematoma. tioning of the carina. Multiple contusions in the thalami and pons were found but POSTOPERATIVE COURSE the medulla oblongata was spared at After evacuation of the epidural haematoma he necropsy. remained comatose. Repeat brain CT showed a In conclusion, medulla oblongata function new large epidural haematoma in the posterior may persist despite rostrocaudal deterio- fossa with a stellate haematoma in the pons and ration. This comatose state (“medulla newly imaged contusions in both thalami. man”) closely mimics brain death. Neurological examination by one of the ( 2001;70:127–129) J Neurol Neurosurg Psychiatry attending physicians showed lack of conscious- Keywords: brain death; head injury; apnoea test; ness, virtually absent brain stem reflexes (with outcome specific attention to vertical eye movement and blinking), and no motor response to pain. -

Orienting Head Movements Resulting from Electrical Microstimulation of the Brainstem Tegmentum in the Barn Owl
The Journal of Neuroscience, January 1993, 13(l): 351370 Orienting Head Movements Resulting from Electrical Microstimulation of the Brainstem Tegmentum in the Barn Owl Tom Masino and Eric I. Knudsen Department of Neurobiology, Stanford University, Stanford, California 943055401 The size and direction of orienting movements are repre- movement latency, duration, velocity, and size each dem- sented systematically as a motor map in the optic tectum of onstrated dependencies on stimulus amplitude, frequency, the barn owl (du Lac and Knudsen, 1990). The optic tectum and duration. projects to several distinct regions in the medial brainstem The data demonstrate directly that at the level of the mid- tegmentum, which in turn project to the spinal cord (Masino brain tegmentum there exists a three-dimensional Cartesian and Knudsen, 1992). This study explores the hypothesis that representation of head-orienting movements such that hor- a fundamental transformation in the neural representation izontal, vertical, and roll components of movement are en- of orienting movements takes place in the brainstem teg- coded by anatomically distinct neural circuits. The data sug- mentum. Head movements evoked by electrical microstim- gest that in the projection from the optic tectum to these ulation in the brainstem tegmentum of the alert barn owl were medial tegmental regions, the topographic code for orienting cataloged and the sites of stimulation were reconstructed movement that originates in the tectum is transformed into histologically. Movements elicited from the brainstem teg- this Cartesian code. mentum were categorized into one of six different classes: [Key words: optic tectum, superior colliculus, saccadic saccadic head rotations, head translations, facial move- head movement, brainstem tegmentum, interstitial nucleus ments, vocalizations, limb movements, and twitches. -

Neuromodulation in Treatment of Hypertension by Acupuncture: a Neurophysiological Prospective
Vol.5, No.4A, 65-72 (2013) Health http://dx.doi.org/10.4236/health.2013.54A009 Neuromodulation in treatment of hypertension by acupuncture: A neurophysiological prospective Peyman Benharash1, Wei Zhou2* 1Division of Cardiothoracic Surgery, University of California, Los Angeles, USA 2Department of Anesthesiology, University of California, Los Angeles, USA; *Corresponding Author: [email protected] Received 28 February 2013; revised 30 March 2013; accepted 6 April 2013 Copyright © 2013 Peyman Benharash, Wei Zhou. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. ABSTRACT study the effects of acupuncture on the hyper- tensive man. Hypertension is a major public health problem affecting over one billion individuals worldwide. Keywords: Central Nervous System; This disease is the result of complex interac- Electroacupuncture; Neurotransmitter; Brain Stem tions between genetic and life-style factors and the central nervous system. Sympathetic hyper- activity has been postulated to be present in 1. INTRODUCTION most forms of hypertension. Pharmaceutical Hypertension has become a serious public health prob- therapy for hypertension has not been perfected, lem impacting over one billion lives worldwide [1]. At often requires a multidrug regimen, and is as- the turn of this century, 7.6 million deaths were attribut- sociated with adverse side effects. Acupuncture, able to hypertension. The majority of this disease burden a form of somatic afferent nerve stimulation has occurred in working people in low to middle-income been used to treat a host of cardiovascular dis- countries, while its prevalence increases with age and the eases such as hypertension. -

Brainstem Dysfunction in Critically Ill Patients
Benghanem et al. Critical Care (2020) 24:5 https://doi.org/10.1186/s13054-019-2718-9 REVIEW Open Access Brainstem dysfunction in critically ill patients Sarah Benghanem1,2 , Aurélien Mazeraud3,4, Eric Azabou5, Vibol Chhor6, Cassia Righy Shinotsuka7,8, Jan Claassen9, Benjamin Rohaut1,9,10† and Tarek Sharshar3,4*† Abstract The brainstem conveys sensory and motor inputs between the spinal cord and the brain, and contains nuclei of the cranial nerves. It controls the sleep-wake cycle and vital functions via the ascending reticular activating system and the autonomic nuclei, respectively. Brainstem dysfunction may lead to sensory and motor deficits, cranial nerve palsies, impairment of consciousness, dysautonomia, and respiratory failure. The brainstem is prone to various primary and secondary insults, resulting in acute or chronic dysfunction. Of particular importance for characterizing brainstem dysfunction and identifying the underlying etiology are a detailed clinical examination, MRI, neurophysiologic tests such as brainstem auditory evoked potentials, and an analysis of the cerebrospinal fluid. Detection of brainstem dysfunction is challenging but of utmost importance in comatose and deeply sedated patients both to guide therapy and to support outcome prediction. In the present review, we summarize the neuroanatomy, clinical syndromes, and diagnostic techniques of critical illness-associated brainstem dysfunction for the critical care setting. Keywords: Brainstem dysfunction, Brain injured patients, Intensive care unit, Sedation, Brainstem -

Isolated Necrosis of Central Tegmental Tracts Due to Neonatal Hypoxic-Ischemic Encephalopathy: MRI Findings
Journal of Neurology & Stroke Case Report Open Access Isolated necrosis of central tegmental tracts due to neonatal hypoxic-ischemic encephalopathy: MRI findings Abstract Volume 11 Issue 1 - 2021 Perinatal hypoxia is an old entity that still prevails today and may lead to neurological Tomás de Andrade Lourenção Freddi, Luiz sequelae that can go unnoticed until a certain age, generating many costs for public health. In this case report, we demonstrate on magnetic resonance imaging an unusual pattern of Fellipe Curvêlo Ciraulo Santos, Nelson Paes perinatal hypoxia in a preterm 5-month-old infant, involving the central tegmental tracts Fortes Diniz Ferreira, Felipe Diego Gomes and briefly discuss its possible pathophysiology. Dantas Department of Radiology, Hospital do Coração, Brazil Keywords: magnetic resonance imaging, asphyxia, hypoxic-ischemic encephalopathy, tegmentum, neonates, brainstem Correspondence: Tomás de Andrade Lourenção Freddi, Hospital do Coração, 147 Desembargador Eliseu Guilherme Street, São Paulo, SP, 04004-030, Brazil, Tel +5511976059280, Email Received: May 25, 2020 | Published: Febrauary 15, 2021 Abbreviations: MRI, magnetic resonance imaging; HII, that connect the red nucleus and the inferior olivary nucleus, being hypoxic-ischemic injury; FLAIR, fluid-attenuated inversion recovery; part of the dentato-rubro-olivary system, called Guillain–Mollaret CTT, central tegmental tract; VGB, vigabatrin triangle.3–5 Introduction Hypoxic-ischemic injury (HII) is one of the most important causes of encephalopathy in neonates, irrespective of gestational age, and may occur in the uterus or during delivery by different intrapartum conditions. In preterm or very low birth weight infants, brain magnetic resonance imaging (MRI) can demonstrate multiple different findings, which a detailed description is beyond the scope of this article, although being periventricular leukomalacia the most frequent (seen in at least 50% of cases). -

SAY: Welcome to Module 1: Anatomy & Physiology of the Brain. This
12/19/2018 11:00 AM FOUNDATIONAL LEARNING SYSTEM 092892-181219 © Johnson & Johnson Servicesv Inc. 2018 All rights reserved. 1 SAY: Welcome to Module 1: Anatomy & Physiology of the Brain. This module will strengthen your understanding of basic neuroanatomy, neurovasculature, and functional roles of specific brain regions. 1 12/19/2018 11:00 AM Lesson 1: Introduction to the Brain The brain is a dense organ with various functional units. Understanding the anatomy of the brain can be aided by looking at it from different organizational layers. In this lesson, we’ll discuss the principle brain regions, layers of the brain, and lobes of the brain, as well as common terms used to orient neuroanatomical discussions. 2 SAY: The brain is a dense organ with various functional units. Understanding the anatomy of the brain can be aided by looking at it from different organizational layers. (Purves 2012/p717/para1) In this lesson, we’ll explore these organizational layers by discussing the principle brain regions, layers of the brain, and lobes of the brain. We’ll also discuss the terms used by scientists and healthcare providers to orient neuroanatomical discussions. 2 12/19/2018 11:00 AM Lesson 1: Learning Objectives • Define terms used to specify neuroanatomical locations • Recall the 4 principle regions of the brain • Identify the 3 layers of the brain and their relative location • Match each of the 4 lobes of the brain with their respective functions 3 SAY: Please take a moment to review the learning objectives for this lesson. 3 12/19/2018 11:00 AM Directional Terms Used in Anatomy 4 SAY: Specific directional terms are used when specifying the location of a structure or area of the brain. -

Chapter 128: Basic Mechanisms of Sleep: New Evidence On
128 BASIC MECHANISMS OF SLEEP: NEW EVIDENCE ON THE NEUROANATOMY AND NEUROMODULATION OF THE NREM-REM CYCLE EDWARD F. PACE-SCHOTT J. ALLAN HOBSON The 1990s brought a wealth of new detail to our knowledge NREM sleep (noradrenergic, serotonergic, and cholinergic of the brain structures involved in the control of sleep and systems damped), and REM sleep (noradrenergic and sero- waking and in the cellular level mechanisms that orchestrate tonergic systems off, cholinergic system undamped) (1–4). the sleep cycle through neuromodulation. This chapter pre- sents these new findings in the context of the general history Original Reciprocal Interaction Model: An of research on the brainstem neuromodulatory systems and Aminergic-Cholinergic Interplay the more specific organization of those systems in the con- trol of the alternation of wake, non–rapid eye movement The model of reciprocal interaction (5) provided a theoretic (NREM), and REM sleep. framework for experimental interventions at the cellular and Although the main focus of the chapter is on the our molecular level that has vindicated the notion that waking own model of reciprocal aminergic-cholinergic interaction, and REM sleep are at opposite ends of an aminergically we review new data suggesting the involvement of many dominant to cholinergically dominant neuromodulatory more chemically specific neuronal groups than can be ac- continuum, with NREM sleep holding an intermediate po- commodated by that model. We also extend our purview to sition (Fig. 128.1). The reciprocal interaction hypothesis the way in which the brainstem interacts with the forebrain. (5) provided a description of the aminergic-cholinergic in- These considerations inform not only sleep-cycle control terplay at the synaptic level and a mathematic analysis of per se, but also the way that circadian and ultradian rhythms the dynamics of the neurobiological control system. -
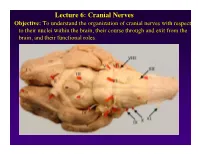
Lecture 6: Cranial Nerves
Lecture 6: Cranial Nerves Objective: To understand the organization of cranial nerves with respect to their nuclei within the brain, their course through and exit from the brain, and their functional roles. Olfactory Eye Muscles 3, 4 &6 Cranial Nerves 1-7 I overview Table, Page 49 II Lecture notes Cranial Nerves and their Functions V Trigeminal VII Facial VIII IX X XII XI Cranial Nerves 8-12 Overview sternocephalic I. Factors Responsible for the Complex Internal Organization of the Brain Stem-> leads to altered location of cranial nerve nuclei in adult brain stem 1. Development of the Fourth Ventricle a. Medulla and Pons develop ventral to the 4th ventricle cerebellum b. Alar plate is displaced lateral to basal plate 4 Medulla Developing Neural Tube 2. Cranial nerve nuclei form discontinuous columns Rostral 12 SE Page 48 Notes 3. Some cranial nerve nuclei migrate from their primitive embryonic positions (e.g., nuclei of V and VII) Facial N. Factors responsible for the complex internal organization of the brainstem: 4) Special senses develop in association with the brain stem. Nuclei of special senses 5) Development of the cerebellum and its connections Cerebellum II. Cranial Nerve Nuclei: Nucleus = column of neuron cell bodies. Efferent nuclei are composed of cell bodies of alpha or gamma motor neurons (SE) or preganglionic parasympathetic neurons (VE). III. Motor Efferent Nuclei (Basal Plate Derivatives): 1. SE (Somatic Efferent) Nuclei: SE neurons form two longitudinally oriented but discontinuous columns of cell bodies in the brain stem. Neurons that comprise these columns are responsible for innervating all of the skeletal musculature of the head. -

Brain Anatomy
BRAIN ANATOMY Adapted from Human Anatomy & Physiology by Marieb and Hoehn (9th ed.) The anatomy of the brain is often discussed in terms of either the embryonic scheme or the medical scheme. The embryonic scheme focuses on developmental pathways and names regions based on embryonic origins. The medical scheme focuses on the layout of the adult brain and names regions based on location and functionality. For this laboratory, we will consider the brain in terms of the medical scheme (Figure 1): Figure 1: General anatomy of the human brain Marieb & Hoehn (Human Anatomy and Physiology, 9th ed.) – Figure 12.2 CEREBRUM: Divided into two hemispheres, the cerebrum is the largest region of the human brain – the two hemispheres together account for ~ 85% of total brain mass. The cerebrum forms the superior part of the brain, covering and obscuring the diencephalon and brain stem similar to the way a mushroom cap covers the top of its stalk. Elevated ridges of tissue, called gyri (singular: gyrus), separated by shallow groves called sulci (singular: sulcus) mark nearly the entire surface of the cerebral hemispheres. Deeper groves, called fissures, separate large regions of the brain. Much of the cerebrum is involved in the processing of somatic sensory and motor information as well as all conscious thoughts and intellectual functions. The outer cortex of the cerebrum is composed of gray matter – billions of neuron cell bodies and unmyelinated axons arranged in six discrete layers. Although only 2 – 4 mm thick, this region accounts for ~ 40% of total brain mass. The inner region is composed of white matter – tracts of myelinated axons.