Dissertationes Kinesiologiae Universitatis Tartuensis 19
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

KERLI MOOSES Physical Activity and Sedentary Time of 7–13 Year-Old
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by DSpace at Tartu University Library KERLI MOOSES DISSERTATIONES KINESIOLOGIAE UNIVERSITATIS TARTUENSIS 44 Physical activity Estonian year-old students Physical in and different segments sedentary day school time 7–13 of KERLI MOOSES Physical activity and sedentary time of 7–13 year-old Estonian students in different school day segments and compliance with physical activity recommendations Tartu 2017 1 ISSN 1406-1058 ISBN 978-9949-77-469-2 DISSERTATIONES KINESIOLOGIAE UNIVERSITATIS TARTUENSIS 44 DISSERTATIONES KINESIOLOGIAE UNIVERSITATIS TARTUENSIS 44 KERLI MOOSES Physical activity and sedentary time of 7–13 year-old Estonian students in different school day segments and compliance with physical activity recommendations Institute of Sport Sciences and Physiotherapy, Faculty of Medicine, University of Tartu, Estonia Dissertation was accepted for the commencement of the Degree of Doctor of Philosophy in Exercise and Sport Sciences on 12th of May 2017 by the Council of the Institute of Sport Sciences and Physiotherapy, University of Tartu, Estonia. Supervisors: Lecturer Merike Kull, PhD Professor Priit Kaasik, PhD Opponent: Research director Tuija Tammelin, PhD LIKES Research Centre for Physical Activity and Health Commencement: Senate Room of the University of Tartu, Ülikooli St. 18, Tartu on 23rd of August 2017, at 12.15 ISSN 1406-1058 ISBN 978-9949-77-469-2 (print) ISBN 978-9949-77-470-8 (pdf) Copyright: Kerli Mooses, 2017 University of Tartu Press www.tyk.ee CONTENTS LIST OF ORIGINAL PUBLICATIONS ....................................................... 6 INTRODUCTION .......................................................................................... 7 1. LITERATURE REVIEW .......................................................................... 8 1.1. The associations between health outcomes, physical activity and sedentary behaviour in children ........................................................ -

Peking 2008 – Mis Eristab Võitjaid Kaotajatest?
Käesolev kogumik on osa Tartu Ülikooli projektist “Tipptreenerite rahvusvaheliste koolituskonverentside korraldamine ja õppematerjalide väljaandmine” Projekti rahastab Euroopa Sotsiaalfond riikliku arengukava meetme 1.1 “Tööjõu paindlikkust, toimetulekut ja elukestvat õpet tagav ning kõigile kättesaadav haridussüsteem” raames. © Tartu Ülikooli kehakultuuriteaduskond Koostaja: Tõnis Matsin Eesti treenerite konverents PEKING 2008 – MIS ERISTAB VÕITJAID KAOTAJATEST? 2. – 3. november 2007 TÜ raamatukogu konverentsikeskus Saateks Intellektuaalne alge on tänapäeva spordis märgatavalt olulisem, kui see võistlusolukorras välja paistab. Naudib ju spordisõber võistlustel sportlase kehalist tublidust, musklite jõudu, kaaslastest liigutuste ilus, kiiruses ja tehnikas üleolekut. Just see paneb vaimustuma, tekitab pinget ning põnevust. Sportlase ajadefitsiidi tingimustes lahendatud mõtlemisülesanded, tema hingeseisund ja motiveeritus jäävad pealtvaatajate eest varju. Nii nagu jääb spordihuviliste enamusele varjatuks see vaimne loominguline protsess, milles osalevad sportlase ja treeneri kõrval sporditeadlased, meedikud, toitumisspetsialistid ja paljud teised asjatundjad ning mille eesmärgiks on kollektiivse mõistuse abil töötada välja optimaalne strateegia ja taktika, parimad meetodid sportlase valmistumisel suurvõistlusteks. “Peking 2008 – mis eristab võitjaid kaotajatest?”, kus esinejateks Eesti olümpiakandidaadid Pekingi olümpiamängudeks, meie silmapaistvad treenerid, sporditeadlased ning -eksperdid, püüab paotada ust sellesse sporditeadmise, - tunnetuse, -

Dragan Milanovic
SPORT SCIENCE Vol. 7, Issue 2. December 2014. INTERNATIONAL SCIENTIFIC JOURNAL OF KINESIOLOGY Publisher: Faculty of Education – University of Travnik, Bosnia & Herzegovina Print ISSN 1840-3662, Web ISSN 1840-3670 UDK 796, Catalogued in: COBISS BH Editor-in-Chief: DAMIR AHMIĆ (Travnik, B&H) Consultant: NIHAD SELIMOVIĆ (Travnik, B&H) Executive Editor: AMRA TUZOVIĆ (Travnik, B&H) Scientific Adviser: OSMO BAJRIĆ (Travnik, B&H) Public relations: MERSIHA BEGANOVIĆ (Travnik, B&H) Indexed in: 'CAB Abstracts', 'CABI Leisure Recreation and Tourism Abstracts', 'CABI Leisure Tourism Database', 'CABI Global Health', 'CABI Nutrition Abstracts and Reviews Series A: Human and Experimental', 'SafetyLit', 'FSO', 'CABI Abstracts on Hygiene and Communicable Diseases', 'Elsevier Scopus', 'Open-J Gate', 'getCITED','ProQuest CSA Physical Education Index', 'ProQuest CSA Natural Sciences', 'ProQuest CSA Social Sciences' 'Genamics Journal Seek', 'Electronic Journals Index (SJSU)', 'Directory of Open Access Journals (DOAJ)', 'EBSCO SPORTDiscus with Full Text', 'EBSCO TOC Premier', 'EBSCO Current Abstracts', 'Index Copernicus', Editorial Board Dragan Milanovic (Zagreb,Croatia), Franja Fratrić (Novi Sad, Serbia), Žarko Kostovski (Skoplje, Macedonia), Djordje Stefanovic (Belgrade, Serbia), Abas Asadi (Iran), Izet Rađo (Sarajevo, BiH), Andrej Švent (Ljubljana, Slovenien), Zvezdan Savić (Nis, Srbija),Branimir Mikić (Travnik, BiH), Edita Kastratović (Belgrade, Serbia), Ifet Mahmutović (Sarajevo, BiH),Branimir Inić ( Belgrade, Serbien), Damir Ahmić (Travnik, BiH), Violeta -

Local Players Have Also the Right to Play
OFFICIAL BULLETIN No. 21 November 2007 Edition in English FÉDÉRATION INTERNATIONALE DE VOLLEYBALL Local players have also the right to play No less than four players affili- The FIVB is considering a proposal to ated to the local Federation and no require that no more than three play- more than two players with a ers with a transfer certificate issued by a different Federation of origin transfer certificate issued by a dif- may be registered by a Volleyball Club ferent Federation of origin to be and only two of them could be on the admitted on the court. A proposal court playing at the same time. The to the next Board of the FIVB. rule will apply to all Clubs involved in National, Continental and/or World The Rally Point System was a Copernican Competitions. revolution, bringing the sideout-era to a modern sport with a more suitable game This proposal has been made after timing for TV and live spectators. Now numerous complaints from National the year 2008 could see Volleyball facing Federations who face difficulties in another outstanding step into the future. finding enough experienced players for It could be a new revolution for the sport, their National teams and its purpose is especially because the FIVB is the first to support and facilitate the access of The FIVB President, Dr. Rubén Acosta H. International Sport Organization to face young players coming from the local frontally the problem of national sport sport programmes to top Volleyball identity. competitions and their participation in stronger Leagues, like Serie A in Italy, National and Inter- have most of their clubs with a few play- national Volleyball ers originally promoted from the local Clubs competitions sport system and too many athletes com- and consequently ing from other National Federations. -
Highest Scoring & Longest Matches
2010 Media Guide Federation Internationale de Volleyball [email protected] Page 112 of 186 SWATCH FIVB World Tour - Highest Scoring Men’s First Set Winning Team ..........................................................Score Losing Team.............................................................Score Location.................................Date......... Total Javier Bosma/Alex Ortiz, Spain.......................................40 Rui Oliveira/Tato Fernandes, Portugal............................38 Espinho.......................... 7/13/2005.............. 78 Andrew Schacht/Joshua Slack, Australia........................37 Taichi Morikawa/Tatsuo Yamamoto, Japan ....................35 Espinho.......................... 6/15/2006.............. 72 Jian Li/Shun Zhou, China ................................................37 Daniel Krug/Mischa Urbatzka, Germany.........................35 Montréal......................... 7/12/2006.............. 72 Jonas Reckermann/Mischa Urbatzka, Germany.............36 Renato "Geor" Gomes/Jorge "Gia" Terceiro, Georgia ....34 Montréal........................... 7/6/2007.............. 70 Tarjei Skarlund/Jarle Huseby, Norway............................35 Alexander Loberg/Bjorn Ingeborgrud, Norway................33 Cadiz................................ 8/7/2002.............. 68 Stephane Canet/Mathieu Hamel, France........................35 Francisco Alvarez/Juan Rossell, Cuba ...........................33 Espinho.......................... 7/26/2003.............. 68 Pedro Brazao/Fred Souza, Brazil....................................35 -

Women'skeihan/Smfg Japan Open Men'svip Open
Women’sRound 4, Men’s Round 5 Fédération Internationale de Volleyball [email protected] Telephone +41-21 345 35 35 Women’s Keihan/Smfg Japan Open Nakanoshima-park, Nakanoshima Kita-ku, Osaka, Japan Men’s VIP Open Lake Jarun, Zagreb, Croatia May 20-25, 2008 Created on May 16, 2008, this fact sheet provides information on the FIVB and the 2008 SWATCH FIVB World Tour. The 2008 SWATCH FIVB World Tour is scheduled over a nine- month period starting in March in Australia and ending in November in China. The US$175,000 Keihan/Smfg Japan Open is the second women’s single gender event of the season while the $175,000 VIP Open is the men’s third event without their female counterparts. Both events will be played May 20-25. After opening the season in South Australia for the inaugural Adelaide Australia Open (March 25-30), the SWATCH FIVB World Tour returns to Osaka for the first-time since the 2005 season. Zagreb hosts a men’s event for the fourth-consecutive season. In addition to Adelaide and Osaka, other new sites on the 2008 SWATCH FIVB World Tour are set for Guaruja (Brazil), Prague (Czech Republic), Myslowice (Poland), Moscow (Russia), Barcelona and Mallorca (Spain), Dubai (United Arab Emirates) and Sanya (China). For more information regarding the SWATCH FIVB World Tour, please visit www.fivb.org. TABLE OF CONTENTS ..............................................................................................................Page · Seoul Open Facts ........................................................................................................................2 -

Xpress 126 English.Indd
No 126 May 2004 English edition FÉDÉRATION INTERNATIONALE DE VOLLEYBALL Qualification Process for Olympic Volleyball Competition Completed The Netherlands men’s team claimed the 12th and final ticket for the Volleyball competition at the 2004 Olympics in Athens after beating hosts Spain to win the World Olympic Qualification tournament in Madrid at the end of May. The tournament in Spain was the last tournament offering Olympic qualifi- cation tickets following a seven-month process for both men and women start- ing in November 2003 with the World Cup in Japan. With hosts Greece already qualified, 11 Olympic tickets were up for grabs for each gender, with three going to the medalists at the World Cup, one ticket each going to the winner of the Continen- tal Olympic qualification tournaments at the start of the year and four more on offer at the World Olympic qualification tournaments in May. The Netherlands rejoice having confirmed their qualification for the Olympic Games In the men’s competition Brazil, Italy and Serbia and Montenegro finished on the podium at the World Cup while Russia, Olympic qualification tournaments in ification ticket as the Asian Continental USA, Argentina and Tunisia won their Portugal and Spain respectively while Olympic qualifier, which went to second respective Continental Olympic quali- France won the World Olympic qualifica- placed Australia. fication tournaments. Poland and the tion tournament in Tokyo, Japan, which Netherlands were victorious at the World also offered a Continental Olympic qual- In the women’s draw, China, Brazil and USA claimed the top three spots at Definitive 12 Women’s Definitive 12 Men’s the World Cup while Germany, Cuba, Olympic qualification berths Olympic qualification berths Dominican Republic and Kenya won their respective Continental Olympic 1. -

China Shanghai Jinshan Open Men'smedal Match Notes
China Shanghai Jinshan Open Men’s Medal Match Notes Fédération Internationale de Volleyball [email protected] Telephone +41-21 345 35 35 Gold Alison Cerutti/Harley Marques, Brazil vs. Pedro Cunha/Pedro Salgado, Brazil Team Uniform Uniform Seed Player No. Player No. Country 1 Alison CeruttI 1 Harley Marques 2 Brazil 2 Pedro Cunha 1 Pedro Salgado 2 Brazil This is the first SWATCH FIVB WORLD TOUR meeting between the two teams. On the Brazilian domestic tour, Alison and Harley have defeated the Pedros twice. · Alison and Harley are playing together for the fist-time on the SWATCH FIVB World Tour since the fall of 2006 when the Brazilians were eliminated during the country quota playoff rounds in Vitoria and placed ninth in Acapulco. · Alison and Harley placed second in the 2009 SWATCH FIVB World Tour season opener in Brasila after losing the finale to Olympic medal winners Emanuel Rego and Ricardo Santos · Harley and Pedro Salgado won nine SWATCH FIVB World Tour gold medals, including 2008 stops in Australia, China, Italy, Switzerland, Mallorca and Brazil. · Harley and Salgado won the 2008 SWATCH FIVB World Tour point’s title. · Harley was the 2008 SWATCH FIVB World Tour most outstanding player. · Harley has also won SWATCH FIVB World Tour gold medals with Para Ferreira (2002 Berlin), Franco Neto (2003 Berlin) and Benjamin Insfran (2005 Montreal and 2008 Sanya). · Alison won a 2008 SWATCH FIVB World Tour gold medal with Pedro Cunha in Bahrain. · On the Brazilian Tour this season for four events, Alison and Harley have a 15-5 match mark with two “final four” finishes and two medals. -

Medal Match Notes
The Hague Open Beach Volleyball 2009 Men’sAugust 30 Schedule Fédération Internationale de Volleyball [email protected] Telephone +41-21 345 35 35 Match Time Court Team A Team B Round 61 14:07 1 Kais Kr.-Vesik EST [10] Harley-Alison BRA [2] Bronze 62 15:37 1 Nummerdor-Schuil NED [1] Herrera-Gavira ESP [3] Gold 59 11:07 1 Nummerdor-Schuil NED [1] Kais Kr.-Vesik EST [10] Semi-finals 60 12:07 1 Herrera-Gavira ESP [3] Harley-Alison BRA [2] Semi-finals Today’s medal matches concludes men’s event No. 242 on the SWATCH FIVB World Tour. Today’s gold medal match is the first SWATCH FIVB World Tour finale between The Netherlands and Spain Today’s gold medal match is the 13th All-European SWATCH FIVB World Tour finale between two European teams, and the 11th between different countries. Here are the past results: 1. Jan Kvalheim/Bjorn Maaseide, NORWAY def. Jean-Philippe Jodard/Christian Penigaud, FRANCE, France - Marseille (7/31/1994), 12-8, 7-12, 15-10, (NA), SWATCH stop No. 26 2. Patrick Heuscher/Stefan Kobel, SWITZERLAND def. Markus Dieckmann/Jonas Reckermann, GERMANY, Switzerland - Gstaad (6/20/2004), 21-18, 27-25 (54 minutes), No. 154 3. M. Dieckmann/Reckermann, GERMANY def. Martin Laciga/Paul Laciga, SWITZERLAND, Germany - Berlin (6/27/2004, 20-22, 21-19, 16-14 (66 minutes), No. 155 4. M. Dieckmann/Reckermann, GERMANY def. Heuscher/Kobel, SWITZERLAND, Brazil -Rio de Janeiro (9/26/2004), 21-18, 19-21, 15-9 (60), No. 162 (ALL EUROPEAN PODIUM, SWITZERLAND THIRD) 5. -
July 20 Update
July 20 Update Fédération Internationale de Volleyball [email protected] Telephone +41-21 345 35 35 2009 SWATCH FIVB World Tour Men’s & Women’s Highlights & Schedule Highlighted by the SWATCH FIVB World Championships and four Grand Slam events, the 2009 SWATCH FIVB World Tour calendar began in April and concludes in November. Here are 2009 SWATCH SWATCH FIVB World Tour highlights. · The total prize money for 2009 is US$7.39-million. · The calendar features 19 sites in 15 countries within three FIVB confederations (Asia, Europe and South America). · The 2009 calendar includes 12 double gender events. · Italy (Rome and Roseto degli Abruzzi) and, Poland (Myslowice) have hosted or will stage men’s only events. · Japan (Osaka), Korea (Seoul), Spain (Barcelona) and Thailand (Phuket) have hosted or will stage women’s only events. · The 2009 SWATCH FIVB World Championships were held June 25-July 5 in Stavanger, Norway. It marked the fifth-time that a European country has hosted the event. · The 2009 calendar includes four SWATCH-FIVB World Tour Grand Slam events with total prize money of $2.4-million. The Swiss Alps village of Gstaad, which hosted the 2007 SWATCH FIVB World Championships, was the first Grand Slam event followed by stops in Moscow, Marseille and Klagenfurt. · The 2009 SWATCH FIVB World Tour calendar lists three new sites, including Brasília (Brazil), Rome (Italy) and The Hague (Netherlands). · The Finnish event in Aland returns to the 2009 SWATCH FIVB World Tour calendar after not being held in 2008 due to the Beijing Olympic Games. · Myslowice hosted a men’s SWATCH FIVB World Tour stop in 2009 after staging a women’sevent in 2008. -

Women'sround6,Men'sround7 GE Money Bank Mazury Open Hotel Anders, Stare Jablonki, Poland June 2-8, 2008
Women’s Round 6, Men’s Round 7 Fédération Internationale de Volleyball [email protected] Telephone +41-21 345 35 35 GE Money Bank Mazury open Hotel Anders, Stare Jablonki, Poland June 2-8, 2008 Updated on June 1, 2008, this fact sheet provides information on the FIVB and the 2008 SWATCH FIVB World Tour. The 2008 SWATCH FIVB World Tour is scheduled over a nine- month period starting in March in Australia and ending in November in China. The US$350,000 GE Money Bank Mazury Open is the fourth of 16 double gender events on the 2008 SWATCH FIVB World Tour calendar. The women’sGE Money Bank Mazury Open competition starts Monday and concludes Saturday (June 7) while the men’s schedule began Tuesday and ends June 8. After opening the season in South Australia for the inaugural Adelaide Australia Open (March 25-30), the SWATCH FIVB World Tour returns to Stare Jablonki for the fifth-straight year. The fifth annual GE Money Bank Mazury Open will also feature women’s competition for the first- time. In addition to Adelaide, other new sites on the 2008 SWATCH FIVB World Tour are set for Guaruja (Brazil), Prague (Czech Republic), Osaka (Japan), Myslowice (Poland), Moscow (Russia), Barcelona and Mallorca (Spain), Dubai (United Arab Emirates) and Sanya (China). For more information regarding the SWATCH FIVB World Tour, please visit www.fivb.org. TABLE OF CONTENTS ...........................................................................................................Page · Hyundai Open Barcelona Facts ............................................................................................. 2 · FIVB Officials........................................................................................................................... 2 · 2008 SWATCH FIVB World Tour Schedule Notes................................................................. 3 · Beijing 2008 Olympic Games ................................................................................................. 3 · GE Money Bank Mazury Open Men’sUpdated Entries through June 1, 2008 ................... -
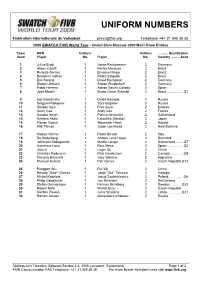
Uniform Numbers
UNIFORM NUMBERS Fédération Internationale de Volleyball [email protected] Telephone +41-21 345 35 35 2009 SWATCH FIVB World Tour - Grand Slam Moscow 2009 Main Draw Entries Team MEN Uniform Uniform ........... Qualification Seed Player No. Player No. Country .......... Seed 1 Julius Brink 1 Jonas Reckermann 2 Germany 2 Alison Cerutti 1 Harley Marques 2 Brazil 3 Ricardo Santos 1 Emanuel Rego 2 Brazil 4 Benjamin Insfran 1 Pedro Salgado 2 Brazil 5 Eric Koreng 1 David Klemperer 2 Germany 6 Stefan Uhmann 1 Stefan Windscheif 2 Germany 7 Pablo Herrera 1 Adrian Gavira Collado 2 Spain 8 Joao Maciel 1 Bruno Oscar Schmidt 2 Brazil ................. Q1 9 Igor Kolodinsky 1 Dmitri Barsouk 2 Russia 10 Serguei Prokopiev 1 Yury Bogatov 2 Russia 11 Kristjan Kais 1 Rivo Vesik 2 Estonia 12 Kevin Ces 1 Andy Ces 2 France 13 Sascha Heyer 1 Patrick Heuscher 2 Switzerland 14 Kentaro Asahi 1 Katsuhiro Shiratori 2 Japan 15 Florian Gosch 1 Alexander Horst 2 Austria 16 Kirk Pitman 1 Jason Lochhead 2 New Zealand 17 Matteo Varnier 1 Paolo Nicolai 2 Italy 18 Bo Soderberg 1 Anders Lund Hoyer 2 Denmark 19 Jefferson Bellaguarda 1 Martin Laciga 2 Switzerland........ Q7 20 Inocencio Lario 1 Raul Mesa 2 Spain ................. Q2 21 Jialu Li 1 Linyin Xu 2 China 22 Christian Redmann 1 Rich VanHuizen 2 Canada.............. Q8 23 Mariano Baracetti 1 Jose Salema 2 Argentina 24 Premysl Kubala 1 Petr Benes 2 Czech RepublicQ14 25 Penggen Wu 1 Rui Yin 2 China 26 Renato "Geor" Gomes 1 Jorge "Gia" Terceiro 2 Georgia 27 Michal Kadziola 1 Jakub Szalankiewicz 2 Poland ..............