19 Diseases of the Placenta Deborah J
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
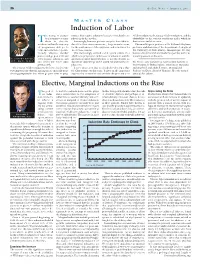
Induction of Labor
36 O B .GYN. NEWS • January 1, 2007 M ASTER C LASS Induction of Labor he timing of parturi- nancies that require induction because of medical com- of labor induction, the timing of labor induction, and the tion remains a conun- plications in the mother. advisability of the various conditions under which in- Tdrum in obstetric Increasingly, however, patients are apt to have labor in- duction can and does occur. medicine in that the majority duced for their own convenience, for personal reasons, This month’s guest professor is Dr. William F. Rayburn, of pregnancies will go to for the convenience of the physician, and sometimes for professor and chairman of the department of ob.gyn. at term and enter labor sponta- all of these reasons. the University of New Mexico, Albuquerque. Dr. Ray- neously, whereas another This increasingly utilized social option ushers in a burn is a maternal and fetal medicine specialist with a na- portion will go post term and whole new perspective on the issue of induction, and the tional reputation in this area. E. ALBERT REECE, often require induction, and question is raised about whether or not the elective in- M.D., PH.D., M.B.A. still others will enter labor duction of labor brings with it added risk and more com- DR. REECE, who specializes in maternal-fetal medicine, is prematurely. plications. Vice President for Medical Affairs, University of Maryland, The concept of labor induction, therefore, has become It is for this reason that we decided to develop a Mas- and the John Z. -

OB/GYN EMERGENCIES Elyse Watkins, Dhsc, PA-C, DFAAPA DISCLOSURES
OB/GYN EMERGENCIES Elyse Watkins, DHSc, PA-C, DFAAPA DISCLOSURES I have no financial relationships to disclose. TOPICS Ovarian torsion Postpartum hemorrhage Ruptured ectopic Acute uterine inversion pregnancy Amniotic fluid embolism Acute menorrhagia Placental abruption OVARIAN TORSION OVARIAN TORSION .Tumors (benign and malignant) are implicated in 50-60% of cases of torsion .20% occur during pregnancy (corpus luteum cyst) .Unilateral or bilateral abdominal-pelvic pain, usually sudden onset .Exercise or movement exacerbates pain .Nausea and vomiting 70% .Pathophys: reduced venous return, stromal edema, internal hemorrhage, and infarction → necrosis OVARIAN TORSION .Physical exam variable .Ultrasonography with color Doppler .Surgical referral RUPTURED ECTOPIC PREGNANCY RUPTURED ECTOPIC PREGNANCY .All patients of reproductive age with a hx of missed menses and pelvic pain should be considered to have an ectopic pregnancy until proven otherwise. .A patient with missed menses, irregular vaginal bleeding, pelvic pain, syncope, abdominal pain, and/or dizziness should be managed as a ruptured ectopic pregnancy until proven otherwise. RUPTURED ECTOPIC PREGNANCY .Physical exam of pts with a ruptured ectopic can reveal pelvic tenderness, an adnexal mass, and evidence of hemodynamic compromise. .A transvaginal ultrasound will often show an adnexal mass and/or fluid in the pouch of Douglas. .The serum qualitative βHCG will be > 5 mIu/mL. RUPTURED ECTOPIC PREGNANCY HTTPS://YOUTU.BE/TNN1FPWHOXS RUPTURED ECTOPIC PREGNANCY .Immediately order an H/H, type and cross, and place large bore IV access for fluid support. .Laparotomy is performed when patients are hemodynamically unstable or if visualization during laparoscopy was difficult. .Patients with a ruptured ectopic pregnancy must be managed emergently and surgically! ACUTE MENORRHAGIA ACUTE MENORRHAGIA .Abnormal uterine bleeding (AUB) can result in acute blood loss that causes hemodynamic compromise so prompt evaluation of vital signs is important. -

Outcomes of Labour of Nuchal Cord
wjpmr, 2020,6(8), 07-15 SJIF Impact Factor: 5.922 Research Article Ansam et al. WORLD JOURNAL OF PHARMACEUTICAL World Journal of Pharmaceutical and Medical Research AND MEDICAL RESEARCH ISSN 2455-3301 www.wjpmr.com WJPMR OUTCOMES OF LABOUR OF NUCHAL CORD Dr. Ansam Layth Abdulhameed*1 and Dr. Rozhan Yassin Khalil2 1Specialist Obstetrics & Gynaecology, Mosul, Iraq. 2Consultant Obstetrics & Gynaecology, Sulaymania, Iraq. *Corresponding Author: Dr. Ansam Layth Abdulhameed Specialist Obstetrics & Gynaecology, Mosul, Iraq. Article Received on 26/05/2020 Article Revised on 16/06/2020 Article Accepted on 06/07/2020 ABSTRACT Background: The nuchal cord is described as the umbilical cord around the fetal neck. It is classified as simple or multiple, loose or tight with the compression of the fetal neck. The term nuchal cord represents an umbilical cord that passes 360 degrees around the fetal neck. Objective: To find out perinatal outcomes in cases of labour of babies with nuchal cord and compared with other cases without nuchal cord. Patient and Methods: The prospective case-control study was conducted in maternity teaching hospital centre in Sulaimani / Kurdistan Region of Iraq, from June 2018 to April 2019. Cases of study divided into two groups. First group comprised of women in whom nuchal cord was present at the time of delivery they were labelled as cases. Second group was a control group composed of women in whom nuchal cord was absent at the time of delivery. Results: This study included (200) patients, (100) women with nuchal cord in labour. (59%) of the cases of nuchal cord in age group (20-29) years, (40%) of them were primigravida, delivery modes for women with nuchal cord were mainly normal vaginal delivery (76%) and cesarean section (24%). -

Porphyromonas Gingivalis Within Placental Villous Mesenchyme and Umbilical Cord Stroma Is Associated with Adverse Pregnancy Outcome
RESEARCH ARTICLE Porphyromonas gingivalis within Placental Villous Mesenchyme and Umbilical Cord Stroma Is Associated with Adverse Pregnancy Outcome Sizzle F. Vanterpool1,2, Jasper V. Been1,3,4, Michiel L. Houben5, Peter G. J. Nikkels6, Ronald R. De Krijger7, Luc J. I. Zimmermann1,8, Boris W. Kramer1,2,8, Ann Progulske-Fox9, Leticia Reyes9,10* 1 Department of Pediatrics, Maastricht University Medical Center, Maastricht, the Netherlands, 2 School for Mental Health and Neurosciences (MHeNS), Maastricht University, Maastricht, the Netherlands, 3 School for Public Health and Primary Care (CAPHRI), Maastricht University, Maastricht, the Netherlands, 4 Division of Neonatology, Erasmus University Medical Center–Sophia Children’s Hospital, Rotterdam, the Netherlands, 5 Department of Pediatrics, Wilhelmina Children’s Hospital, University Medical Center Utrecht, Utrecht, the Netherlands, 6 Department of Pathology, University Medical Center Utrecht, Utrecht, the Netherlands, 7 Department of Pathology, Erasmus University Medical Center, Rotterdam, the Netherlands, 8 School for Oncology and Developmental Biology (GROW), Maastricht University, Maastricht, the Netherlands, OPEN ACCESS 9 Department of Oral Biology, Center for Molecular Microbiology, University of Florida, Gainesville, Florida, Citation: Vanterpool SF, Been JV, Houben ML, United States of America, 10 Department of Pathobiological Sciences, University of Wisconsin-Madison, Nikkels PGJ, De Krijger RR, Zimmermann LJI, et al. Madison, Wisconsin, United States of America (2016) Porphyromonas gingivalis within Placental * [email protected] Villous Mesenchyme and Umbilical Cord Stroma Is Associated with Adverse Pregnancy Outcome. PLoS ONE 11(1): e0146157. doi:10.1371/journal. pone.0146157 Abstract Editor: Motohiro Komaki, Tokyo Medical and Dental University, JAPAN Intrauterine presence of Porphyromonas gingivalis (Pg), a common oral pathobiont, is impli- cated in preterm birth. -

Gestational Diabetes Insipidus, HELLP Syndrome and Eclampsia in a Twin Pregnancy: a Case Report
Journal of Perinatology (2010) 30, 144–145 r 2010 Nature Publishing Group All rights reserved. 0743-8346/10 $32 www.nature.com/jp PERINATAL/NEONATAL CASE PRESENTATION Gestational diabetes insipidus, HELLP syndrome and eclampsia in a twin pregnancy: a case report JL Woelk, RA Dombroski and PR Brezina Department of Obstetrics and Gynecology, East Carolina University, Greenville, NC, USA alanine aminotransferase, 65 U lÀ1; and lactate dehydrogenase, We report a case of eclampsia in a twin pregnancy complicated by HELLP 390 U lÀ1. A 24-h urine collection was begun to quantify proteinuria. syndrome and diabetes insipidus. This confluence of disease processes During the first night of hospitalization, the patient developed suggests that a modification of common magnesium sulfate treatment marked polyuria and polydipsia. Urine output increased to 1500 cc hÀ1 protocols may be appropriate in a certain subset of patients. by the early morning totaling 12 l for the entire night. Repeat Journal of Perinatology (2010) 30, 144–145; doi:10.1038/jp.2009.115 electrolytes were unchanged. The endocrinology service was then Keywords: diabetes insipidus ; eclampsia ; HELLP syndrome ; twin consulted for the management of presumptive DI. Therapy with the pregnancy ; magnesium sulfate administration of dDAVP (1-deamino-8-D-arginine vasopressin) orally twice daily was initiated. Serum osmolality was increased at 296 mOsm kgÀ1, and urine osmolality was decreased to 71 mOsm kgÀ1 Introduction with a specific gravity of 1.000. The 24-h urine results showed a total The association between diabetes insipidus (DI) and liver protein of 780 mg. The patient denied the previously reported dysfunction in a preeclamptic patient has been established in headaches, blurry vision and right upper quadrant pain, and her blood multiple case reports.1 This case is an important example that pressures remained 140 to 155 mmHg (systolic) and 80 to 95 mmHg illustrates how the pathophysiology of DI and the pharmacokinetics (diastolic), consistent with a diagnosis of mild preeclampsia. -

Uterine Atony: Uterus Soft and Relaxed
Uterine atony: uterus soft and relaxed Placenta not delivered Treat for whole retained placenta If whole placenta still retained ■ Oxytocin ■ Manual removal with prophylactic antibiotics ■ Controlled cord traction ■ Intraumbilical vein injection (if no bleeding) Placenta delivered incomplete Treat for retained placenta fragments If bleeding continues ■ Oxytocin ■ Manage as uterine atony ■ Manual exploration to remove fragments ■ Gentle curettage or aspiration Be ready at all times to transfer to a higher-level facility if the Lower genital tract trauma: Treat for lower genital tract trauma If bleeding continues patient is not responding to the excessive bleeding or shock ■ Repair of tears ■ Tranexamic acid ■ treatment or a treatment cannot contracted uterus Evacuation and repair of haematoma be administered at your facility. Uterine rupture or dehiscence: Treat for uterine rupture or dehiscence If bleeding continues excessive bleeding or shock ■ Laparotomy for primary repair of uterus ■ Tranexamic acid Start intravenous oxytocin infusion ■ Hysterectomy if repair fails and consider: • uterine massage; • bimanual uterine compression; Uterine inversion: Treat for uterine inversion If laparotomy correction not successful • external aortic compression; and uterine fundus not felt ■ Immediate manual replacement ■ Hysterectomy • balloon or condom tamponade. abdominally or visible in vagina ■ Hydrostatic correction ■ Manual reverse inversion Transfer with ongoing intravenous (use general anaesthesia or wait for effect uterotonic infusion. Accompanying -

In This Issue
THE JOURNAL OF THE AAPA VOLUME 8 ISSUE 4 2018 IN THIS ISSUE Peer-Reviewed 1 CE Quiz & Peer-Reviewed Manuscript: New Malignant Transformation of Childhood Malignant Transformation of Burn Wound with Metastasis: A Case CE Article Report Childhood Burn Wound with 3 Letter from the Editor Metastasis: A Case Report N. Dominic Alessio, PA(ASCP)CM 6 CE Quiz & Peer-Reviewed Manuscript: Breast Cancer Metastasis to the Colon Detroit Medical Center, Detroit, MI Presenting After Fifteen Years Fellow members were given the opportunity to apply for a travel grant to attend an upcoming Fall Conference or Spring Meeting of 8 Peer-Reviewed Manuscript: their choice. Fellows were required to write a manuscript, and the Aurora Diagnostics Pathologists’ Assistant four winning entries received a grant valued at up to $1800 (full week Breast Specimen Handling Best Practice Guideline registration + $1000 to help cover travel expenses). Congratulations, Dominic, on your winning submission! 11 44th Annual Continuing Education Conference Recap Abstract 12 44th Annual Continuing Education Marjolin’s ulcer is a rare and aggressive form of cutaneous squamous cell carcinoma Conference Photos (SCC) which forms through malignant transformation of chronically irritated previous injury, such as incompletely healed burns, ulcers, and other wounds. Although similar in 17 8th Annual Spring Meeting microscopic morphology, Marjolin’s ulcer is unique from other cutaneous SCCs in many other significant characteristics. The carcinoma often appears decades after the initial 18 Board of Trustees Chair’s Report trauma, but once present it follows a rapid course of growth and metastasis. In the current case study, a male in his mid-30s with history of extensive burns as a child presented 21 Gross Photo Unknown to the Emergency Department complaining of a large, open wound on his lower back. -

ABCDE Acronym Blood Transfusion 231 Major Trauma 234 Maternal
Cambridge University Press 978-0-521-26827-1 - Obstetric and Intrapartum Emergencies: A Practical Guide to Management Edwin Chandraharan and Sir Sabaratnam Arulkumaran Index More information Index ABCDE acronym albumin, blood plasma levels 7 arterial blood gas (ABG) 188 blood transfusion 231 allergic anaphylaxis 229 arterio-venous occlusions 166–167 major trauma 234 maternal collapse 12, 130–131 amiadarone, overdose 178 aspiration 10, 246 newborn infant 241 amniocentesis 234 aspirin 26, 180–181 resuscitation 127–131 amniotic fluid embolism 48–51 assisted reproduction 93 abdomen caesarean section 257 asthma 4, 150, 151, 152, 185 examination after trauma 234 massive haemorrhage 33 pain in pregnancy 154–160, 161 maternal collapse 10, 13, 128 atracurium, drug reactions 231 accreta, placenta 250, 252, 255 anaemia, physiological 1, 7 atrial fibrillation 205 ACE inhibitors, overdose 178 anaerobic metabolism 242 automated external defibrillator (AED) 12 acid–base analysis 104 anaesthesia. See general anaesthesia awareness under anaesthesia 215, 217 acidosis 94, 180–181, 186, 242 anal incontinence 138–139 ACTH levels 210 analgesia 11, 100, 218 barbiturates, overdose 178 activated charcoal 177, 180–181 anaphylaxis 11, 227–228, 229–231 behaviour/beliefs, psychiatric activated partial thromboplastin time antacid prophylaxis 217 emergencies 172 (APTT) 19, 21 antenatal screening, DVT 16 benign intracranial hypertension 166 activated protein C 46 antepartum haemorrhage 33, 93–94. benzodiazepines, overdose 178 Addison’s disease 208–209 See also massive -

Blood Volume in Newborn Piglets: Effects of Time of Natural Cord Rupture, Intra-Uterine Growth Retardation, Asphyxia, and Prostaglandin-Induced Prematurity
Pediatr. Res. 15: 53-57 (1981) asphyxia natural cord rupture blood volume prostaglandin F 2 intra-uterine growth retardation Blood Volume in Newborn Piglets: Effects of Time of Natural Cord Rupture, Intra-Uterine Growth Retardation, Asphyxia, and Prostaglandin-Induced Prematurity 137 OTWIN LINDERKAMP, , KLAUS BETKE, MONIKA GUNTNER, GIOK H. JAP, KLAUS P. RIEGEL, AND KURT WALSER Department of Pediatrics and Department of Veterinary Gynecology, University of Munich, Munich, Federal Republic of Germany Summary (27, 29, 32). Placental transfusion is accelerated by keeping the infant below the placenta (19, 27), by uterine contractions (32), Blood volume (BV), red cell mass (RCM; Cr-51) and plasma 125 and by respiration of the newborn (19). Placental transfusion is volume ( 1-labeled albumin) were measured in lOS piglets from prevented by holding the infant above the placenta (19, 27), by 28 Utters shortly after birth. Spontaneous cord rupture in healthy maternal hypotension ( 17), by tight nuchal cord ( 13), and by acute piglets occurred during delivery (n • 25) or within 190 sec of birth intrapartum asphyxia (5, 12, 13). Intra-uterine asphyxia results in (n • 82). Spontaneous and induced delay of cord rupture resulted prenatal transfusion to the fetus (12, 13, 33). In a time-dependent Increase in BV and RCM. BV (x ± S.D.) at It is to be assumed that blood volume in newborn mammals is birth was 72.5 ± 10.5 ml/kg (RCM, 23.6 ± 4.6 ml/kg) In the 25 similarly influenced by placental transfusion as in the human piglets with prenatal cord rupture and 110.5 ± 12.9 ml/kg (RCM, neonate. -

Incidence of Eclampsia with HELLP Syndrome and Associated Mortality in Latin America
International Journal of Gynecology and Obstetrics 129 (2015) 219–222 Contents lists available at ScienceDirect International Journal of Gynecology and Obstetrics journal homepage: www.elsevier.com/locate/ijgo CLINICAL ARTICLE Incidence of eclampsia with HELLP syndrome and associated mortality in Latin America Paulino Vigil-De Gracia a,⁎, José Rojas-Suarez b, Edwin Ramos c, Osvaldo Reyes d, Jorge Collantes e, Arelys Quintero f,ErasmoHuertasg, Andrés Calle h, Eduardo Turcios i,VicenteY.Chonj a Critical Care Unit, Department of Obstetrics and Gynecology, Complejo Hospitalario de la Caja de Seguro Social, Panama City, Panama b Critical Care Unit, Clínica de Maternidad Rafael Calvo, Cartagena, Colombia c Department of Gynecology and Obstetrics, Hospital Universitario Dr Luis Razetti, Barcelona, Venezuela d Unit of Research, Department of Gynecology and Obstetrics, Hospital Santo Tomás, Panama City, Panama e Department of Gynecology and Obstetrics, Hospital Regional de Cojamarca, Cajamarca, Peru f Department of Gynecology and Obstetrics, Hospital José Domingo de Obaldía, David, Panama g Unit of Perinatology, Department of Gynecology and Obstetrics, Instituto Nacional Materno Perinatal, Lima, Peru h Department of Gynecology and Obstetrics, Hospital Carlos Andrade Marín, Quito, Ecuador i Unit of Research, Department of Gynecology and Obstetrics, Hospital Primero de Mayo de Seguridad Social, San Salvador, El Salvador j Department of Gynecology and Obstetrics, Hospital Teodoro Maldonado Carbo, Guayaquil, Ecuador article info abstract Article history: Objective: To describe the maternal outcome among women with eclampsia with and without HELLP syndrome Received 7 July 2014 (hemolysis, elevated liver enzymes, and low platelet count). Methods: A cross-sectional study of women with Received in revised form 14 November 2014 eclampsia was undertaken in 14 maternity units in Latin America between January 1 and December 31, 2012. -

Is Nuchal Cord a Cause of Concern?
ISSN: 2638-1575 Madridge Journal of Women’s Health and Emancipation Research Article Open Access Is Nuchal Cord a cause of concern? Surekha Tayade1*, Jaya Kore1, Atul Tayade2, Neha Gangane1, Ketki Thool1 and Jyoti Borkar1 1Department of Obstetrics and Gynecology, Mahatma Gandhi Institute of Medical Sciences, Sewagram, India 2Department of Radiodiagnosis, Mahatma Gandhi Institute of Medical Sciences, Sewagram, India Article Info Abstract *Corresponding author: Context: The controversy about whether nuchal cord is a cause of concern and its Surekha Tayade adverse effect on perinatal outcome still persists. Authors express varying views and Professor Department of Obstetrics and Gynecology hence managing pregnancy with cord around the neck has its own concerns. Thus study Mahatma Gandhi Institute of Medical was carried out to find out the incidence of nuchal cord and its implications. Sciences Sewagram, 442102 Method: This was a prospective, cross sectional, comparative study carried out in the India Kasturba Hospital of MGIMS, Sewagram a rural medical tertiary care institute. All Tel: +917887519832 deliveries over a period of one year, were enrollled and studied for nuchal cord, tight or E-mail: [email protected] loose cord, number of turns, fetal heart rate irregulaties, pregnancy and perinatal Received: May 15, 2018 outcome. Accepted: June 19, 2018 Results: Total women with nuchal cord in labour room were 1116 (2.56%) of which Published: June 23, 2018 85.21 percent had single turn around the babies neck. Most of the babies ( 77.96 %) had Citation: Tayade S, Kore J, Tayade A, Gangane a loose nuchal cord, however 22.04 percent had a tight cord. -

Umbilical Cord Prolapse Guideline
Umbilical Cord Prolapse Guideline Document Control Title Umbilical Cord Prolapse Guideline Author Author’s job title Specialty Trainee in Obstetrics and Gynaecology Directorate Department Women’s and Children’s Obstetrics and Gynaecology Date Version Status Comment / Changes / Approval Issued 1.0 Mar Final Approved by the Maternity Services Guideline Group in 2011 April 2011. 1.1 Aug Revision Minor amendments by Corporate Governance to 2012 document control report, headers and footers, new table of contents, formatting for document map navigation. 2.0 Feb Final Approved by the Maternity Services Guideline Group in 2016 February 2016. 2.1 Apr Revision Harmonised with Royal Devon & Exeter guideline 2019 3.0 May Final Approved by Maternity Specialist Governance Forum 2019 meeting on 01.05.2019 Main Contact ST1 O&G Tel: Direct Dial– 01271 311806 North Devon District Hospital Raleigh Park Barnstaple Devon EX31 4JB Lead Director Medical Director Superseded Documents Nil Issue Date Review Date Review Cycle May 2019 May 2022 Three years Consulted with the following stakeholders: (list all) Senior obstetricians Senior midwives Senior management team Filename Umbilical Cord Prolapse Guideline v3. 01May 19.doc Policy categories for Trust’s internal Tags for Trust’s internal website (Bob) website (Bob) Cord, accidents, prolapse Maternity Services Maternity Page 1 of 11 Umbilical Cord Prolapse Guideline CONTENTS Document Control .................................................................................................... 1 1. Introduction