Summary of Product Characteristics
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Opioid-Induced Hyperalgesia in Humans Molecular Mechanisms and Clinical Considerations
SPECIAL TOPIC SERIES Opioid-induced Hyperalgesia in Humans Molecular Mechanisms and Clinical Considerations Larry F. Chu, MD, MS (BCHM), MS (Epidemiology),* Martin S. Angst, MD,* and David Clark, MD, PhD*w treatment of acute and cancer-related pain. However, Abstract: Opioid-induced hyperalgesia (OIH) is most broadly recent evidence suggests that opioid medications may also defined as a state of nociceptive sensitization caused by exposure be useful for the treatment of chronic noncancer pain, at to opioids. The state is characterized by a paradoxical response least in the short term.3–14 whereby a patient receiving opioids for the treatment of pain Perhaps because of this new evidence, opioid may actually become more sensitive to certain painful stimuli. medications have been increasingly prescribed by primary The type of pain experienced may or may not be different from care physicians and other patient care providers for the original underlying painful condition. Although the precise chronic painful conditions.15,16 Indeed, opioids are molecular mechanism is not yet understood, it is generally among the most common medications prescribed by thought to result from neuroplastic changes in the peripheral physicians in the United States17 and accounted for 235 and central nervous systems that lead to sensitization of million prescriptions in the year 2004.18 pronociceptive pathways. OIH seems to be a distinct, definable, One of the principal factors that differentiate the use and characteristic phenomenon that may explain loss of opioid of opioids for the treatment of pain concerns the duration efficacy in some cases. Clinicians should suspect expression of of intended use. -

Opioids): SOR/2018-77 Canada Gazette, Part II, Volume 152, Number 9
Canada Gazette, Part 2, Volume 152, Number 9: Regulations Amendin... http://gazette.gc.ca/rp-pr/p2/2018/2018-05-02/html/sor-dors77-eng.html Home (http://www.canada.ca/en/index.html) How government works (http://www.canada.ca/en/government/system/index.html) Treaties, laws and regulations (https://www.canada.ca/en/government/system/laws.html) Canada Gazette (/accueil-home-eng.html) Publications (/rp-pr/publications-eng.html) Part II: Vol. 152 (2018) (/rp-pr/p2/2018/index-eng.html) May 2, 2018 (/rp-pr/p2/2018/2018-05-02/html/index-eng.html) Regulations Amending the Food and Drug Regulations (Opioids): SOR/2018-77 Canada Gazette, Part II, Volume 152, Number 9 Registration April 23, 2018 FOOD AND DRUGS ACT P.C. 2018-429 April 20, 2018 Her Excellency the Governor General in Council, on the recommendation of the Minister of Health, pursuant to section 30a of the Food and Drugs Act b, makes the annexed Regulations Amending the Food and Drug Regulations (Opioids). Regulations Amending the Food and Drug Regulations (Opioids) Amendments 1 Subsections C.01.005(2) and (3) of the Food and Drug Regulations 1 are replaced by the following: (2) Subsection (1) does not apply to (a) a drug in dosage form that is compounded by a pharmacist under a prescription or by a practitioner; or (b) a drug in dosage form that is sold under a prescription if the following information appears on the drug’s label: (i) the drug’s proper name, common name or brand name, (ii) the drug’s potency, and (iii) the name of the drug’s manufacturer. -

Opioid Tolerance in Methadone Maintenance Treatment: Comparison
Gutwinski et al. Harm Reduction Journal (2016) 13:7 DOI 10.1186/s12954-016-0095-0 RESEARCH Open Access Opioid tolerance in methadone maintenance treatment: comparison of methadone and levomethadone in long-term treatment Stefan Gutwinski*, Nikola Schoofs, Heiner Stuke, Thomas G. Riemer, Corinde E. Wiers and Felix Bermpohl Abstract Background: This study aimed to investigate the development of opioid tolerance in patients receiving long-term methadone maintenance treatment (MMT). Methods: A region-wide cross-sectional study was performed focusing on dosage and duration of treatment. Differences between racemic methadone and levomethadone were examined. All 20 psychiatric hospitals and all 110 outpatient clinics in Berlin licensed to offer MMT were approached in order to reach patients under MMT fulfilling the DSM IV criteria of opiate dependence. In the study, 720 patients treated with racemic methadone or levomethadone gave information on the dosage of treatment. Out of these, 679 patients indicated the duration of MMT. Results: Treatment with racemic methadone was reported for 370 patients (54.5 %), with levomethadone for 309 patients (45.5 %). Mean duration of MMT was 7.5 years. We found a significant correlation between dosage and duration of treatment, both in a conjoint analysis for the two substances racemic methadone and levomethadone and for each substance separately. These effects remained significant when only patients receiving MMT for 1 year or longer were considered, indicating proceeding tolerance development in long-term treatment. When correlations were compared between racemic methadone and levomethadone, no significant difference was found. Conclusions: Our data show a tolerance development under long-term treatment with both racemic methadone and levomethadone. -

Clinical and Pharmacokinetic Evaluation of S
Casoni et al. Acta Veterinaria Scandinavica (2015) 57:21 DOI 10.1186/s13028-015-0112-4 RESEARCH Open Access Clinical and pharmacokinetic evaluation of S-ketamine for intravenous general anaesthesia in horses undergoing field castration Daniela Casoni1*, Claudia Spadavecchia1, Beat Wampfler2, Wolfgang Thormann3 and Olivier L Levionnois1 Abstract Background: Intravenous anaesthetic drugs are the primary means for producing general anaesthesia in equine practice. The ideal drug for intravenous anaesthesia has high reliability and pharmacokinetic properties indicating short elimination and lack of accumulation when administered for prolonged periods. Induction of general anaesthesia with racemic ketamine preceded by profound sedation has already an established place in the equine field anaesthesia. Due to potential advantages over racemic ketamine, S-ketamine has been employed in horses to induce general anaesthesia, but its optimal dose remains under investigation. The objective of this study was to evaluate whether 2.5 mg/kg S-ketamine could be used as a single intravenous bolus to provide short-term surgical anaesthesia in colts undergoing surgical castration, and to report its pharmacokinetic profile. Results: After premedication with romifidine and L-methadone, the combination of S-ketamine and diazepam allowed reaching surgical anaesthesia in the 28 colts. Induction of anaesthesia as well as recovery were good to excellent in the majority (n = 22 and 24, respectively) of the colts. Seven horses required additional administration of S-ketamine to prolong the duration of surgical anaesthesia. Redosing did not compromise recovery quality. Plasma concentration of S-ketamine decreased rapidly after administration, following a two-compartmental model, leading to the hypothesis of a consistent unchanged elimination of the parent compound into the urine beside its conversion to S-norketamine. -

Opioid-Induced Hyperalgesia a Qualitative Systematic Review Martin S
Anesthesiology 2006; 104:570–87 © 2006 American Society of Anesthesiologists, Inc. Lippincott Williams & Wilkins, Inc. Opioid-induced Hyperalgesia A Qualitative Systematic Review Martin S. Angst, M.D.,* J. David Clark, M.D., Ph.D.† Opioids are the cornerstone therapy for the treatment of an all-inclusive and current overview of a topic that may moderate to severe pain. Although common concerns regard- be difficult to grasp as a whole because new evidence ing the use of opioids include the potential for detrimental side accumulates quickly and in quite distinct research fields. effects, physical dependence, and addiction, accumulating evi- dence suggests that opioids may yet cause another problem, As such, a comprehensive review may serve as a source often referred to as opioid-induced hyperalgesia. Somewhat document. However, a systematic review also uses a paradoxically, opioid therapy aiming at alleviating pain may framework for presenting information, and such a frame- Downloaded from http://pubs.asahq.org/anesthesiology/article-pdf/104/3/570/360792/0000542-200603000-00025.pdf by guest on 01 October 2021 render patients more sensitive to pain and potentially may work may facilitate and clarify future communication by aggravate their preexisting pain. This review provides a com- clearly delineating various entities or aspects of OIH. prehensive summary of basic and clinical research concerning opioid-induced hyperalgesia, suggests a framework for organiz- Finally, a systematic review aims at defining the status ing pertinent information, delineates the status quo of our quo of our knowledge concerning OIH, a necessary task knowledge, identifies potential clinical implications, and dis- to guide future research efforts and to identify potential cusses future research directions. -

RMP Levopidon
VI.2 Elements for a public summary VI.2.1 Overview of disease epidemiology Levomethadone is indicated as substitution therapy for maintenance of opioid dependence in adults in conjunction with appropriate medical, social and psychosocial care. Opioid users entering specialist treatment are on average 33 years old, with female patients being younger in most countries. Across Europe, male opioid clients outnumber their female counterparts by a ratio of about three to one. The great majority of opioid clients report having started to use the drug before the age of 30, with almost half (46 %) of all opioid clients having done so before the age of 20. In general, opioid users report higher levels of homelessness and unemployment and lower levels of education than primary users of other drugs, and they are usually concentrated in urban areas. Source: EMCDDA (European Monitoring Centre for Drugs and Drug Addiction) 2012 Annual report on the state of the drugs problem in Europe http://www.emcdda.europa.eu/publications/annual-report/2012 VI.2.2 Summary of treatment benefits Levomethadone reduces withdrawal symptoms in people addicted to heroin or other narcotic drugs without causing the “high” associated with the drug addiction. Levomethadone treatment is an integral part of detoxification and maintenance programs in case of opioid addiction. There are several relevant objectives of levomethadone maintenance therapy: to suppress signs and symptoms of opioid withdrawal, to extinguish opioid-drug craving, and to block the reinforcing effects of illicit opioids. Each of these objectives is accomplished in phases, rather than at once, relying on the administration of adequate levomethadone doses to achieve and sustain optimum blood levels. -

Oklahoma Drug Threat Assessment
2018 Oklahoma Drug Threat Assessment Oklahoma Bureau of Narcotics and Dangerous Drugs i John Scully, Director MARY FALLIN JOHN SCULLY Governor Director September 12, 2018 The Oklahoma Bureau of Narcotics has served the citizens of Oklahoma in the quest for a drug- free state since 1975. Our agency remains committed to working with lawmakers, law enforcement, public health officials, and the citizens of Oklahoma to develop comprehensive strategies to address drug abuse in communities across the state. While we know many factors contribute to drug abuse, OBN is committed to reducing the availability of illegal drugs in Oklahoma. Our agency works to eradicate illegal drugs in Oklahoma by enforcing drug laws, administering statewide programs, and providing continual outreach to our stakeholders – lawmakers, law enforcement, public health officials, and the citizens of Oklahoma. OBN agents enforce drug laws by utilizing aggressive investigative methods and administering statewide drug diversion programs. The Prescription Drug Monitoring Program is a valuable tool for practitioners, pharmacists, and law enforcement in the prevention and detection of the diversion and abuse of pharmaceutical controlled substances. As a service to our communities, OBN also administers the Safe Trips for Scripts Drug Prevention Program. This program provides citizens a safe way to discard unwanted medications by disposing of them in one of our 177 take back boxes located around the state. This drug threat assessment was created to help provide officials and citizens with helpful information about drug threats across our state. We will continue to collaborate with other agencies and the citizens to work toward a safer and healthier Oklahoma. -
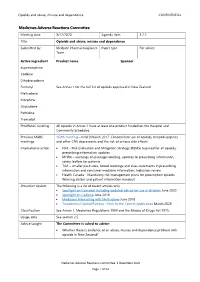
3.2.2 Opioids and Abuse, Misuse and Dependence
Opioids and abuse, misuse and dependence CONFIDENTIAL Medicines Adverse Reactions Committee Meeting date 3/12/2020 Agenda item 3.2.2 Title Opioids and abuse, misuse and dependence Submitted by Medsafe Pharmacovigilance Paper type For advice Team Active ingredient Product name Sponsor Buprenorphine Codeine Dihydrocodeine Fentanyl See Annex 1 for the full list of opioids approved in New Zealand Methadone Morphine Oxycodone Pethidine Tramadol PHARMAC funding All opioids in Annex 1 have at least one product funded on the Hospital and Community Schedules. Previous MARC 169th meeting – held 9 March 2017: Concomitant use of opioids, benzodiazepines meetings and other CNS depressants and the risk of serious side effects International action • FDA - Risk Evaluation and Mitigation Strategy (REMS) required for all opioids; prescribing information updates • MHRA – warnings on package labelling, updates to prescribing information, safety leaflets for patients • TGA – smaller pack sizes, boxed warnings and class statements in prescribing information and consumer medicine information, indication review • Health Canada – Mandatory risk management plans for prescription opioids, Warning sticker and patient information handout Prescriber Update The following is a list of recent articles only. • Spotlight on tramadol including updated advice for use in children June 2020 • Spotlight on Codeine June 2018 • Medicines Interacting with Methadone June 2018 • Transdermal Opioid Patches - Stick to the Correct Application March 2020 Classification See Annex 1. Medicines -

Patterns of Opioid Prescribing in Minnesota: 2012 and 2015
ISSUE BRIEF | APRIL, 2018 Patterns of Opioid Prescribing in Minnesota: 2012 and 2015 Introduction Key Findings: Opioids are a class of drugs that include prescription • Overall rates of opioid prescribing declined in opioid medications for pain relief —such as oxycodone Minnesota from 2012 to 2015, but the morphine (OxyContin®), hydrocodone (Vicodin®), codeine, morphine, milligram equivalents (MME) per prescription and fentanyl—as well as illicitly produced drugs like heroin increased. and fentanyl-related substances (also called fentanyl analogs).1 While prescription opioids play a role in the • Medicare and Medicaid, where eligibility is determined management of some types of severe acute, cancer-related by age, disability status, and/or income, covered and end-of-life pain, increased opioid use since 1990, approximately one-third of Minnesotans with general including for chronic pain unrelated to cancer, has resulted health coverage and accounted for two-thirds of opioid in sharply rising opioid addiction and overdoses, as well as prescriptions filled in 2015. increased healthcare utilization and costs. Recent Centers • Nearly one in three Minnesotans with an opioid for Disease Control and Prevention (CDC) guidelines point prescription in 2015 had multiple prescribers. out the limitations of the evidence base in support of opioid therapy for pain, recommend non-opioid therapy • In both 2012 and 2015, 6 in 10 opioid prescriptions for chronic pain, and emphasize the risks associated with were filled within 15 days of the patient’s last medical opioid therapy.2 In Minnesota, opioids—both prescription visit; however, 1 in 10 opioid prescriptions were filled and illicit—were responsible for 336 overdose deaths without a medical visit in the past 90 days, suggesting in 2015, more than a six-fold increase since 2000.3 In closer patient-prescriber communication or opioid 2016, opioid use accounted for 395 overdose deaths in oversight may be needed in some cases. -

HPLC Enantioseparation of Tramadol and Its Metabolites: Method Validation and Application to Environmental Samples
HPLC enantioseparation of Tramadol and its metabolites: method validation and application to environmental samples Cátia Sofia Rodrigues Silva Master in Quality Control Faculty of Pharmacy of University of Porto 2016 Separação enantiomérica por HPLC do Tramadol e seus metabolitos: validação do método e aplicação em amostras ambientais Cátia Sofia Rodrigues Silva Dissertação do 2º Ciclo de Estudos Conducente ao Grau de Mestre em Controlo de Qualidade – especialização em Ambiente Trabalho realizado sob a orientação do Professor Doutor Carlos Manuel Magalhães Afonso e Professora Doutora Maria Elizabeth Tiritan 2016 De acordo com a legislação em vigor, não é permitida a reprodução de qualquer parte desta dissertação/tese HPLC enantioseparation of tramadol and metabolites: method validation and application to environmental samples ACKNOWLEDGEMENTS I would like to thank to Prof. Dr. Carlos Afonso, my supervisor, and Prof.ª Dr.ª Maria Elizabeth Tiritan, my co-supervision, for all the support, patience and dedication during the realization of this seminar and the experimental work. Your unconditional support was very important to me and it was certainly a privilege to work with you and acquire all knowledge you have given to me. To Prof.ª Dr.ª Cláudia Ribeiro, to Master Alexandra Maia, PhD Student, and to Master Virgínia Gonçalves, Superior technical of IINFACTS, I would like to thank for all the support that given me in experimental work, over this year, and for all the attention and availability in the laboratory’s work. To my parents, my family and my friends which have a lot of patience for me and make me laugh in the certain time. -

Perioperative Immunosuppression and Risk of Cancer Progression: the Impact of Opioids on Pain Management
Hindawi Pain Research and Management Volume 2018, Article ID 9293704, 8 pages https://doi.org/10.1155/2018/9293704 Review Article Perioperative Immunosuppression and Risk of Cancer Progression: The Impact of Opioids on Pain Management Renata Zaja˛czkowska ,1 Wojciech Leppert,2 Joanna Mika ,3 Magdalena Kocot-Ke˛pska,4 Jarosław Woron´ ,1 Anna Wrzosek ,1 and Jerzy Wordliczek 1 1Department of Interdisciplinary Intensive Care, Jagiellonian University Medical College, Krako´w, Poland 2Department of Palliative Medicine, Poznan University of Medical Sciences, Poznan, Poland 3Department of Pain Pharmacology, Institute of Pharmacology, Polish Academy of Sciences, Krak´ow, Poland 4Department of Pain Research and Treatment, Jagiellonian University Medical College, Krako´w, Poland Correspondence should be addressed to Anna Wrzosek; [email protected] Received 24 May 2018; Revised 5 August 2018; Accepted 19 August 2018; Published 19 September 2018 Academic Editor: Massimiliano Valeriani Copyright © 2018 Renata Zaja˛czkowska et al. -is is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Opioids comprise an important group of drugs used in cancer pain pharmacotherapy. In recent years, more and more studies have emerged indicating the potentially immunosuppressive effects of opioid analgesics and their serious consequences, including the risk of cancer progression. -e identification of these risks has prompted a search for other effective, and most importantly, safer methods of perioperative analgesic management. Regional analgesia techniques, which allow for a significant reduction in opioid dosing and thus diminish the risk of immunosuppression associated with these drugs, seem to offer substantial hope in this respect. -

Opioid Prescription Drug Monitoring Surveillance Report 2015
Opioid Prescription Drug Monitoring Surveillance Report 2015 Department of Health and Human Services Division of Public Health Opioid Prescription Drug Monitoring Surveillance Report Release date: November 28, 2016 Report prepared by: Megan Key, BS Jamie S. White, MPH Melody Law, MD, MPH Olivia Kasirye, MD, MS Sacramento County Department of Health and Human Services Public Health Division Disease Control and Epidemiology Unit 7001‐A East Parkway, Suite 600 Sacramento, CA 95823 Phone: 916‐875‐5881 TTY: 877‐835‐2929 Website: www.scph.com i Opioid Prescription Drug Monitoring Surveillance Report Introduction Contents INTRODUCTION ....................................................................................................................................................................... 1 The Opioid Epidemic ........................................................................................................................................................... 1 Controlled Substance Utilization Review and Evaluation System (CURES) ........................................................................ 1 Prescriber Guidelines .......................................................................................................................................................... 2 Purpose ............................................................................................................................................................................... 2 Acknowledgments ..............................................................................................................................................................