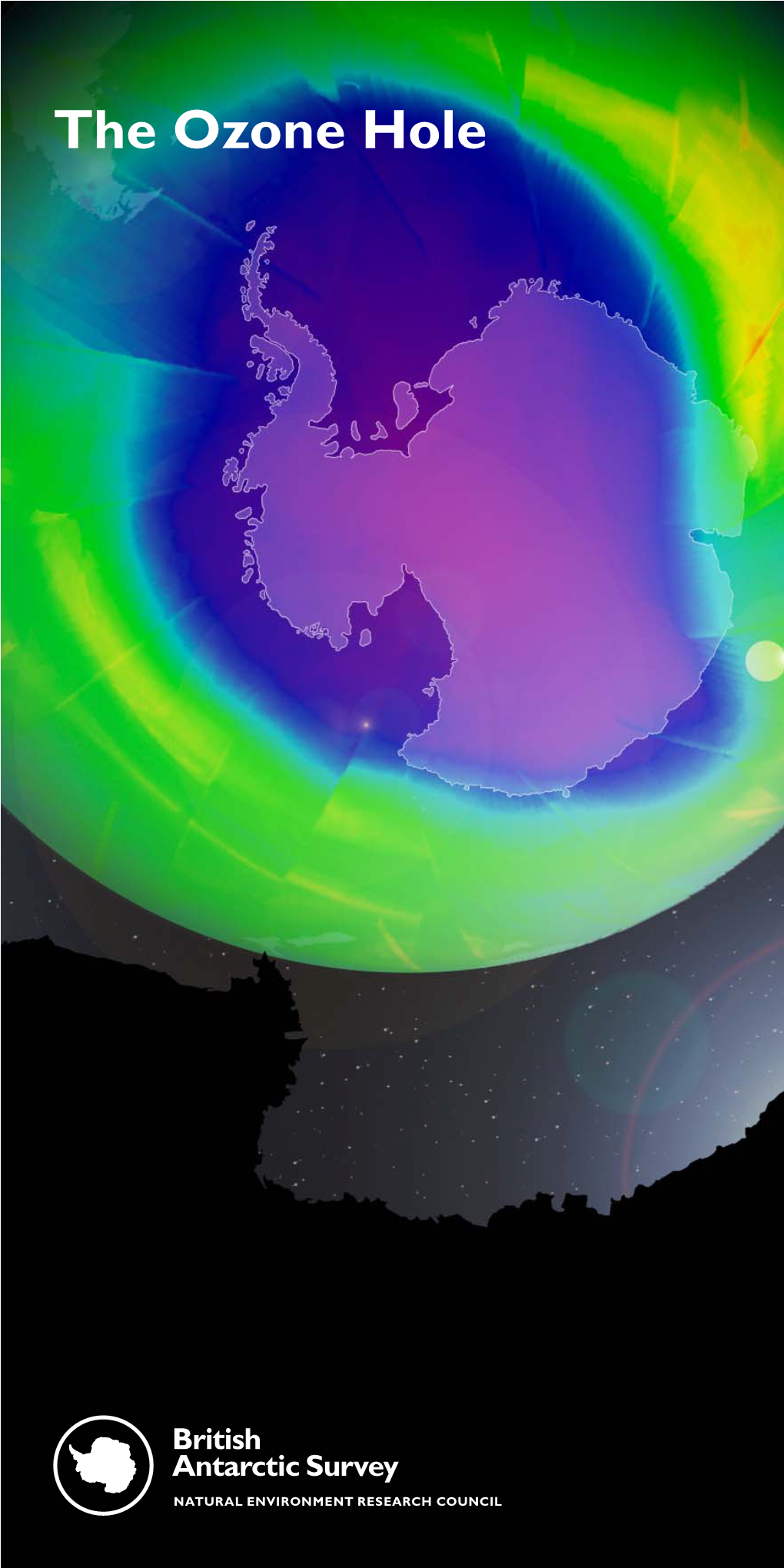
The Ozone Hole High up in the Earth’S Atmosphere a Thin Layer of Ozone Protects Us from the Sun’S Most Harmful Rays, Safeguarding Life on Our Planet
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Lecture 21: Glaciers and Paleoclimate Read: Chapter 15 Homework Due Thursday Nov
Learning Objectives (LO) Lecture 21: Glaciers and Paleoclimate Read: Chapter 15 Homework due Thursday Nov. 12 What we’ll learn today:! 1. 1. Glaciers and where they occur! 2. 2. Compare depositional and erosional features of glaciers! 3. 3. Earth-Sun orbital parameters, relevance to interglacial periods ! A glacier is a river of ice. Glaciers can range in size from: 100s of m (mountain glaciers) to 100s of km (continental ice sheets) Most glaciers are 1000s to 100,000s of years old! The Snowline is the lowest elevation of a perennial (2 yrs) snow field. Glaciers can only form above the snowline, where snow does not completely melt in the summer. Requirements: Cold temperatures Polar latitudes or high elevations Sufficient snow Flat area for snow to accumulate Permafrost is permanently frozen soil beneath a seasonal active layer that supports plant life Glaciers are made of compressed, recrystallized snow. Snow buildup in the zone of accumulation flows downhill into the zone of wastage. Glacier-Covered Areas Glacier Coverage (km2) No glaciers in Australia! 160,000 glaciers total 47 countries have glaciers 94% of Earth’s ice is in Greenland and Antarctica Mountain Glaciers are Retreating Worldwide The Antarctic Ice Sheet The Greenland Ice Sheet Glaciers flow downhill through ductile (plastic) deformation & by basal sliding. Brittle deformation near the surface makes cracks, or crevasses. Antarctic ice sheet: ductile flow extends into the ocean to form an ice shelf. Wilkins Ice shelf Breakup http://www.youtube.com/watch?v=XUltAHerfpk The Greenland Ice Sheet has fewer and smaller ice shelves. Erosional Features Unique erosional landforms remain after glaciers melt. -

Minimal Geological Methane Emissions During the Younger Dryas-Preboreal Abrupt Warming Event
UC San Diego UC San Diego Previously Published Works Title Minimal geological methane emissions during the Younger Dryas-Preboreal abrupt warming event. Permalink https://escholarship.org/uc/item/1j0249ms Journal Nature, 548(7668) ISSN 0028-0836 Authors Petrenko, Vasilii V Smith, Andrew M Schaefer, Hinrich et al. Publication Date 2017-08-01 DOI 10.1038/nature23316 Peer reviewed eScholarship.org Powered by the California Digital Library University of California LETTER doi:10.1038/nature23316 Minimal geological methane emissions during the Younger Dryas–Preboreal abrupt warming event Vasilii V. Petrenko1, Andrew M. Smith2, Hinrich Schaefer3, Katja Riedel3, Edward Brook4, Daniel Baggenstos5,6, Christina Harth5, Quan Hua2, Christo Buizert4, Adrian Schilt4, Xavier Fain7, Logan Mitchell4,8, Thomas Bauska4,9, Anais Orsi5,10, Ray F. Weiss5 & Jeffrey P. Severinghaus5 Methane (CH4) is a powerful greenhouse gas and plays a key part atmosphere can only produce combined estimates of natural geological in global atmospheric chemistry. Natural geological emissions and anthropogenic fossil CH4 emissions (refs 2, 12). (fossil methane vented naturally from marine and terrestrial Polar ice contains samples of the preindustrial atmosphere and seeps and mud volcanoes) are thought to contribute around offers the opportunity to quantify geological CH4 in the absence of 52 teragrams of methane per year to the global methane source, anthropogenic fossil CH4. A recent study used a combination of revised 13 13 about 10 per cent of the total, but both bottom-up methods source δ C isotopic signatures and published ice core δ CH4 data to 1 −1 2 (measuring emissions) and top-down approaches (measuring estimate natural geological CH4 at 51 ± 20 Tg CH4 yr (1σ range) , atmospheric mole fractions and isotopes)2 for constraining these in agreement with the bottom-up assessment of ref. -
Sea Level and Climate Introduction
Sea Level and Climate Introduction Global sea level and the Earth’s climate are closely linked. The Earth’s climate has warmed about 1°C (1.8°F) during the last 100 years. As the climate has warmed following the end of a recent cold period known as the “Little Ice Age” in the 19th century, sea level has been rising about 1 to 2 millimeters per year due to the reduction in volume of ice caps, ice fields, and mountain glaciers in addition to the thermal expansion of ocean water. If present trends continue, including an increase in global temperatures caused by increased greenhouse-gas emissions, many of the world’s mountain glaciers will disap- pear. For example, at the current rate of melting, most glaciers will be gone from Glacier National Park, Montana, by the middle of the next century (fig. 1). In Iceland, about 11 percent of the island is covered by glaciers (mostly ice caps). If warm- ing continues, Iceland’s glaciers will decrease by 40 percent by 2100 and virtually disappear by 2200. Most of the current global land ice mass is located in the Antarctic and Greenland ice sheets (table 1). Complete melt- ing of these ice sheets could lead to a sea-level rise of about 80 meters, whereas melting of all other glaciers could lead to a Figure 1. Grinnell Glacier in Glacier National Park, Montana; sea-level rise of only one-half meter. photograph by Carl H. Key, USGS, in 1981. The glacier has been retreating rapidly since the early 1900’s. -

Calving Processes and the Dynamics of Calving Glaciers ⁎ Douglas I
Earth-Science Reviews 82 (2007) 143–179 www.elsevier.com/locate/earscirev Calving processes and the dynamics of calving glaciers ⁎ Douglas I. Benn a,b, , Charles R. Warren a, Ruth H. Mottram a a School of Geography and Geosciences, University of St Andrews, KY16 9AL, UK b The University Centre in Svalbard, PO Box 156, N-9171 Longyearbyen, Norway Received 26 October 2006; accepted 13 February 2007 Available online 27 February 2007 Abstract Calving of icebergs is an important component of mass loss from the polar ice sheets and glaciers in many parts of the world. Calving rates can increase dramatically in response to increases in velocity and/or retreat of the glacier margin, with important implications for sea level change. Despite their importance, calving and related dynamic processes are poorly represented in the current generation of ice sheet models. This is largely because understanding the ‘calving problem’ involves several other long-standing problems in glaciology, combined with the difficulties and dangers of field data collection. In this paper, we systematically review different aspects of the calving problem, and outline a new framework for representing calving processes in ice sheet models. We define a hierarchy of calving processes, to distinguish those that exert a fundamental control on the position of the ice margin from more localised processes responsible for individual calving events. The first-order control on calving is the strain rate arising from spatial variations in velocity (particularly sliding speed), which determines the location and depth of surface crevasses. Superimposed on this first-order process are second-order processes that can further erode the ice margin. -

Comparison of Remote Sensing Extraction Methods for Glacier Firn Line- Considering Urumqi Glacier No.1 As the Experimental Area
E3S Web of Conferences 218, 04024 (2020) https://doi.org/10.1051/e3sconf/202021804024 ISEESE 2020 Comparison of remote sensing extraction methods for glacier firn line- considering Urumqi Glacier No.1 as the experimental area YANJUN ZHAO1, JUN ZHAO1, XIAOYING YUE2and YANQIANG WANG1 1College of Geography and Environmental Science, Northwest Normal University, Lanzhou, China 2State Key Laboratory of Cryospheric Sciences, Northwest Institute of Eco-Environment and Resources/Tien Shan Glaciological Station, Chinese Academy of Sciences, Lanzhou, China Abstract. In mid-latitude glaciers, the altitude of the snowline at the end of the ablating season can be used to indicate the equilibrium line, which can be used as an approximation for it. In this paper, Urumqi Glacier No.1 was selected as the experimental area while Landsat TM/ETM+/OLI images were used to analyze and compare the accuracy as well as applicability of the visual interpretation, Normalized Difference Snow Index, single-band threshold and albedo remote sensing inversion methods for the extraction of the firn lines. The results show that the visual interpretation and the albedo remote sensing inversion methods have strong adaptability, alonger with the high accuracy of the extracted firn line while it is followed by the Normalized Difference Snow Index and the single-band threshold methods. In the year with extremely negative mass balance, the altitude deviation of the firn line extracted by different methods is increased. Except for the years with extremely negative mass balance, the altitude of the firn line at the end of the ablating season has a good indication for the altitude of the balance line. -

A Globally Complete Inventory of Glaciers
Journal of Glaciology, Vol. 60, No. 221, 2014 doi: 10.3189/2014JoG13J176 537 The Randolph Glacier Inventory: a globally complete inventory of glaciers W. Tad PFEFFER,1 Anthony A. ARENDT,2 Andrew BLISS,2 Tobias BOLCH,3,4 J. Graham COGLEY,5 Alex S. GARDNER,6 Jon-Ove HAGEN,7 Regine HOCK,2,8 Georg KASER,9 Christian KIENHOLZ,2 Evan S. MILES,10 Geir MOHOLDT,11 Nico MOÈ LG,3 Frank PAUL,3 Valentina RADICÂ ,12 Philipp RASTNER,3 Bruce H. RAUP,13 Justin RICH,2 Martin J. SHARP,14 THE RANDOLPH CONSORTIUM15 1Institute of Arctic and Alpine Research, University of Colorado, Boulder, CO, USA 2Geophysical Institute, University of Alaska Fairbanks, Fairbanks, AK, USA 3Department of Geography, University of ZuÈrich, ZuÈrich, Switzerland 4Institute for Cartography, Technische UniversitaÈt Dresden, Dresden, Germany 5Department of Geography, Trent University, Peterborough, Ontario, Canada E-mail: [email protected] 6Graduate School of Geography, Clark University, Worcester, MA, USA 7Department of Geosciences, University of Oslo, Oslo, Norway 8Department of Earth Sciences, Uppsala University, Uppsala, Sweden 9Institute of Meteorology and Geophysics, University of Innsbruck, Innsbruck, Austria 10Scott Polar Research Institute, University of Cambridge, Cambridge, UK 11Institute of Geophysics and Planetary Physics, Scripps Institution of Oceanography, University of California, San Diego, La Jolla, CA, USA 12Department of Earth, Ocean and Atmospheric Sciences, University of British Columbia, Vancouver, British Columbia, Canada 13National Snow and Ice Data Center, University of Colorado, Boulder, CO, USA 14Department of Earth and Atmospheric Sciences, University of Alberta, Edmonton, Alberta, Canada 15A complete list of Consortium authors is in the Appendix ABSTRACT. The Randolph Glacier Inventory (RGI) is a globally complete collection of digital outlines of glaciers, excluding the ice sheets, developed to meet the needs of the Fifth Assessment of the Intergovernmental Panel on Climate Change for estimates of past and future mass balance. -

Saving the Arctic the Urgent Need to Cut Black Carbon Emissions and Slow Climate Change
AP PHOTO/IAN JOUGHIN PHOTO/IAN AP Saving the Arctic The Urgent Need to Cut Black Carbon Emissions and Slow Climate Change By Rebecca Lefton and Cathleen Kelly August 2014 WWW.AMERICANPROGRESS.ORG Saving the Arctic The Urgent Need to Cut Black Carbon Emissions and Slow Climate Change By Rebecca Lefton and Cathleen Kelly August 2014 Contents 1 Introduction and summary 3 Where does black carbon come from? 6 Future black carbon emissions in the Arctic 9 Why cutting black carbon outside the Arctic matters 13 Recommendations 19 Conclusion 20 Appendix 24 Endnotes Introduction and summary The Arctic is warming at a rate twice as fast as the rest of the world, in part because of the harsh effects of black carbon pollution on the region, which is made up mostly of snow and ice.1 Black carbon—one of the main components of soot—is a deadly and widespread air pollutant and a potent driver of climate change, espe- cially in the near term and on a regional basis. In colder, icier regions such as the Arctic, it peppers the Arctic snow with heat-absorbing black particles, increasing the amount of heat absorbed and rapidly accelerating local warming. This accel- eration exposes darker ground or water, causing snow and ice melt and lowering the amount of heat reflected away from the Earth.2 Combating climate change requires immediate and long-term cuts in heat-trap- ping carbon pollution, or CO2, around the globe. But reducing carbon pollution alone will not be enough to avoid the worst effects of a rapidly warming Arctic— slashing black carbon emissions near the Arctic and globally must also be part of the solution. -

1 Edited Transcript of a Recording of Jon Shanklin Interviewed by Chris Eldon Lee on the 29Th November 2013. BAS Archives AD6/24
JON SHANKLIN Edited transcript of a recording of Jon Shanklin interviewed by Chris Eldon Lee on the 29th November 2013. BAS Archives AD6/24/1/236. Transcribed by Andy Smith, 5 March 2017. Part One [Part 1 0:00:00] Lee: This is Jon Shanklin, interviewed by Chris Eldon Lee, on the 29 th of November 2013. Jon Shanklin, Part 1. Shanklin: Jonathan Shanklin, born 1953, September the 29 th , in Wrexham, Denbighshire. [Part 1 0:00:18] Lee: Oh right? North Wales? Shanklin: North Wales. [Part 1 0:00:20] Lee: I live in Shrewsbury, so I know. Shanklin: I was in Shrewsbury in September actually. [Part 1 0:00:24] Lee: What, professionally? Shanklin: Amateurly because I am also a botanist. In fact I have just been appointed the Co-recorder for Cambridgeshire and there was a Recorders’ conference in the Gateway. [Part 1 0:00:39] Lee: Would you say your father was an educated man? Shanklin: Yes. [Part 1 0:00:42] Lee: Tell me about him. Shanklin: Dad was a consultant geologist at McAlpines, the big construction company, and so he would go off prospecting all over the place. I remember when I was a child he had several visits to South Africa, when he was away for months at a time. Probably rather like me, going to the Antarctic for months at a time, repaying the compliment, if you like. My mum was also a geologist, but mostly a mother, but they were both members of the local geological society. Dad at one time was a president of it, and they had regular excursions, and as children myself and my brother would be packed into the bus which would go down lanes that were very very narrow. -

Changes in Glacier Facies Zonation on Devon Ice Cap, Nunavut, Detected
The Cryosphere Discuss., https://doi.org/10.5194/tc-2018-250 Manuscript under review for journal The Cryosphere Discussion started: 26 November 2018 c Author(s) 2018. CC BY 4.0 License. 1 Changes in glacier facies zonation on Devon Ice Cap, Nunavut, detected from SAR imagery 2 and field observations 3 4 Tyler de Jong1, Luke Copland1, David Burgess2 5 1 Department of Geography, Environment and Geomatics, University of Ottawa, Ottawa, Ontario 6 K1N 6N5, Canada 7 2 Natural Resources Canada, 601 Booth St., Ottawa, Ontario K1A 0E8, Canada 8 9 Abstract 10 Envisat ASAR WS images, verified against mass balance, ice core, ground-penetrating radar and 11 air temperature measurements, are used to map changes in the distribution of glacier facies zones 12 across Devon Ice Cap between 2004 and 2011. Glacier ice, saturation/percolation and pseudo dry 13 snow zones are readily distinguishable in the satellite imagery, and the superimposed ice zone can 14 be mapped after comparison with ground measurements. Over the study period there has been a 15 clear upglacier migration of glacier facies, resulting in regions close to the firn line switching from 16 being part of the accumulation area with high backscatter to being part of the ablation area with 17 relatively low backscatter. This has coincided with a rapid increase in positive degree days near 18 the ice cap summit, and an increase in the glacier ice zone from 71% of the ice cap in 2005 to 92% 19 of the ice cap in 2011. This has significant implications for the area of the ice cap subject to 20 meltwater runoff. -

The Creation of Global Imaginaries: the Antarctic Ozone Hole and the Isoline Tradition in the Atmospheric Sciences Sebastian Grevsmühl
The Creation of Global Imaginaries: The Antarctic Ozone Hole and the Isoline Tradition in the Atmospheric Sciences Sebastian Grevsmühl To cite this version: Sebastian Grevsmühl. The Creation of Global Imaginaries: The Antarctic Ozone Hole and the Isoline Tradition in the Atmospheric Sciences. Birgit Schneider, Thomas Nocke. Image Politics of Climate Change, Transcript, pp.29-53, 2014, 978-3-8376-2610-0. hal-01519392 HAL Id: hal-01519392 https://hal.archives-ouvertes.fr/hal-01519392 Submitted on 7 May 2017 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Distributed under a Creative Commons Attribution - NonCommercial - ShareAlike| 4.0 International License The Creation of Global Imaginaries: The Antarctic Ozone Hole and the Isoline Tradition in the Atmospheric Sciences (in: Birgit Schneider and Thomas Nocke (eds.), Image Politics of Climate Change, Bielefeld, Transcript, 2014, p.29-53). Sebastian Vincent Grevsmühl Abstract This historical essay retraces from the perspective of visual and material culture how ways of analyzing and visualizing atmospheric -

Issu E 2 5 E a Ste R 2 0 1 3 the Magazine of Corpus Christi College
3 1 0 2 r e t s a E 5 2 e u pelican The magazine of Corpus Christi College Cambridge s s I Baroness Professor The Rt Honourable Lord Hodge Elizabeth Butler-Sloss David Ibbetson Sir Terence Etherton writes about his interviewed by interviewed by interviewed by journey to the Robert McCrum Philip Murray Simon Heffer Supreme Court Page 4 Page 10 Page 14 Page 20 Contents 3 The Master’s Introduction 4 Baroness Elizabeth Butler-Sloss 10 Professor David Ibbetson 14 Sir Terrence Etherton 20 Lord Hodge 24 Joe Farman 28 Dr Pietro Circuta 32 Dr Nicholas Chen 36 A View from the Plodge Editor: Elizabeth Winter Assistant editor: Rowena Bermingham Editorial assistant: Emma Murray Photography: Phil Mynott, Nicholas Sack Produced by Cameron Design 01284 725292 Master’s Introduction I am delighted once more to introduce this issue of the Pelican , brought to you by Liz Winter and the members of our energetic Development team. We try to use the magazine to show the diversity of College life; and this issue is no exception. It has a strong legal flavour, highlighting the lives and achievements of some of Corpus’s lawyers, and demonstrating the strength we have in this subject and in the profession. You will be interested to read the interviews with Baroness Elizabeth Butler-Sloss, our newest Honorary Fellow, and also with Sir Terence Etherton and Lord (Patrick) Hodge; Professor David Ibbetson also features – many of you will know him as Regius Professor of Civil Law and Warden of Leckhampton, shortly to leave us to become President of Clare Hall. -

(Sentinel-2 and Landsat 8) Optical Data
remote sensing Article Cross-Comparison of Albedo Products for Glacier Surfaces Derived from Airborne and Satellite (Sentinel-2 and Landsat 8) Optical Data Kathrin Naegeli 1,*, Alexander Damm 2, Matthias Huss 1,3, Hendrik Wulf 2, Michael Schaepman 2 and Martin Hoelzle 1 1 Department of Geosciences, University of Fribourg, 1700 Fribourg, Switzerland; [email protected] (M.Hu.); [email protected] (M.Ho.) 2 Remote Sensing Laboratories, University of Zurich, 8057 Zurich, Switzerland; [email protected] (A.D.); [email protected] (H.W.); [email protected] (M.S.) 3 Laboratory of Hydraulics, Hydrology and Glaciology (VAW), ETH Zurich, 8093 Zurich, Switzerland * Correspondence: [email protected]; Tel.: +41-026-300-9008 Academic Editors: Frank Paul, Kate Briggs, Robert McNabb, Christopher Nuth, Jan Wuite, Magaly Koch, Xiaofeng Li and Prasad S. Thenkabail Received: 21 October 2016; Accepted: 19 January 2017; Published: 27 January 2017 Abstract: Surface albedo partitions the amount of energy received by glacier surfaces from shortwave fluxes and modulates the energy available for melt processes. The ice-albedo feedback, influenced by the contamination of bare-ice surfaces with light-absorbing impurities, plays a major role in the melting of mountain glaciers in a warming climate. However, little is known about the spatial and temporal distribution and variability of bare-ice glacier surface albedo under changing conditions. In this study, we focus on two mountain glaciers located in the western Swiss Alps and perform a cross-comparison of different albedo products. We take advantage of high spectral and spatial resolution (284 bands, 2 m) imaging spectrometer data from the Airborne Prism Experiment (APEX) and investigate the applicability and potential of Sentinel-2 and Landsat 8 data to derive broadband albedo products.