Kussin-Shoptaw-Thesis-2020
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
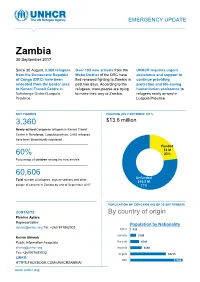
Zambia 30 September 2017
EMERGENCY UPDATE Zambia 30 September 2017 Since 30 August, 3,360 refugees Over 100 new arrivals from the UNHCR requires urgent from the Democratic Republic Moba District of the DRC have assistance and support to of Congo (DRC) have been fled renewed fighting to Zambia in continue providing relocated from the border area past two days. According to the protection and life-saving to Kenani Transit Centre in refugees, more people are trying humanitarian assistance to Nchelenge District/Luapula to make their way to Zambia. refugees newly arrived in Province. Luapula Province. KEY FIGURES FUNDING (AS 2 OCTOBER 2017) 3,360 $13.6 million requested for Zambia operation Newly-arrived Congolese refugees in Kenani Transit Centre in Nchelenge, Luapula province. 2,063 refugees have been biometrically registered Funded $3 M 60% 23% Percentage of children among the new arrivals Unfunded XX% 60,606 [Figure] M Unfunded Total number of refugees, asylum-seekers and other $10.5 M people of concern in Zambia by end of September 2017 77% POPULATION OF CONCERN (AS OF 30 SEPTEMBER) CONTACTS By country of origin Pierrine Aylara Representative Population by Nationality [email protected] Tel: +260 977862002 Other 415 Somalia 3199 Kelvin Shimoh Public Information Associate Burundi 4749 [email protected] Rwanda 6130 Tel: +260979585832 Angola 18715 LINKS: DRC 27398 HTTPS:FACEBOOK.COM/UNHCRZAMBIA/ www.unhcr.org 1 EMERGENCY UPDATE > Zambia / 30 September 2017 Emergency Response Luapula province, northern Zambia Since 30 August, over 3,000 asylum-seekers from the Democratic Republic of Congo (DRC) have crossed into northern Zambia. New arrivals are reportedly fleeing insecurity and clashes between Congolese security forces FARDC and a local militia groups in towns of Pweto, Manono, Mitwaba (Haut Katanga Province) as well as in Moba and Kalemie (Tanganyika Province). -

ZAMBIA HUMANITARIAN SITUATION REPORT 1 January to 30 June 2018
UNICEF ZAMBIA HUMANITARIAN SITUATION REPORT 1 January to 30 June 2018 Zambia Humanitarian Situation Report ©UNICEF Zambia/2017/Ayisi ©UNICEF REPORTING PERIOD: JANUARY - JUNE 2018 SITUATION IN NUMBERS Highlights 15,425 # of registered refugees in Nchelengue • As of 28 June 2018, a total of 15,425 refugees from the district Democratic Republic of the Congo (DRC) were registered at (UNHCR, Infographic 28 June 2018) Kenani transit centre in the Luapula Province of Zambia. • UNICEF and partners are supporting the Government of Zambia 79% to provide life-saving services for all the refugees in Kenani of registered refugees are women and transit centre and in the Mantapala permanent settlement area. children • More than half of the refugees have been relocated to Mantapala permanent settlement area. 25,000 • The set-up of basic services in Mantapala is drastically delayed # of expected new refugees from DRC in due to heavy rainfall that has made access roads impassable. Nchelengue District in 2018 • Discussions between UNICEF and the Government are under way to develop a transition and sustainability plan to ensure the US$ 8.8 million continuity of services in refugee hosting areas. UNICEF funding requirement UNICEF’s Response with Partners Funding Status 2018 UNICEF Sector Carry- forward Total Total amount: UNICEF Sector $0.2 m Funds received current Target Results* Target year: $2.5 m Results* Nutrition: # of children admitted for SAM 400 273 400 273 treatment Health: # of children vaccinated against 11,875 6,690 11,875 6,690 measles WASH: # of people provided with access to 15,000 9,253 25,000 15,425 Funding Gap: $6.1 m safe water =68% Child Protection: # of children receiving 5,500 3,657 9,000 4,668 psychosocial and/or other protection services Funds available include funding received for the current year as well as the carry-forward from the previous year. -

Bridging the Gap Between Household Demand and Community Water Supply?
KUMAMARU, SMOUT & ODHIAMBO 35th WEDC International Conference, Loughborough, UK, 2011 THE FUTURE OF WATER, SANITATION AND HYGIENE: INNOVATION, ADAPTATION AND ENGAGEMENT IN A CHANGING WORLD Self-supply: bridging the gap between household demand and community water supply? K. Kumamaru, I. Smout & F. Odhiambo REFEREED PAPER 1089 This paper discusses rural water supply at grassroots level, and challenges the assumption that a community water supply facility is the only solution for rural water supply, especially in sparsely populated areas. A comparison is made between two water service models from case studies in Zambia: those with conventional communal water supplies and Self Supply models. Findings revealed that a Self Supply service could significantly reduce faecal contamination risk in water quality and deliver higher per capita water use and better convenience of access than conventional supply, yet its reliability regarding water source dry up requires to be monitored. A conventional community-based water service may not fulfil the households’ demand due to the nature of community ownership and the distance from household to a community water facility. Since the underlying service delivery models are different, an integrated approach is required for a rural water supply strategy and national policy. Introduction The priority for sustainable rural water supplies has changed from „providing the physical systems‟ to „delivering the water service‟ over the past few decades. The water sector generates a lot of analyses on costs, designs, post-construction supports, management models, efficiency and sustainability of supplies but these are mostly for conventional communal supply service models (Sutton 2010b). The term „community‟ has no single definition but varies in the context of different view points from the place in which people live, to groups of population, culture, religion and history. -

The Case of Honey in Zambia the Case
Small-scale with outstanding economic potential enterprises woodland-based In some countries, honey and beeswax are so important the term ‘beekeeping’ appears in the titles of some government ministries. The significance of honey and beeswax in local livelihoods is nowhere more apparent than in the Miombo woodlands of southern Africa. Bee-keeping is a vital source of income for many poor and remote rural producers throughout the Miombo, often because it is highly suited to small scale farming. This detailed Non-Timber Forest Product study from Zambia examines beekeeping’s livelihood role from a range of perspectives, including market factors, production methods and measures for harnessing beekeeping to help reduce poverty. The caseThe in Zambia of honey ISBN 979-24-4673-7 Small-scale woodland-based enterprises with outstanding economic potential 9 789792 446739 The case of honey in Zambia G. Mickels-Kokwe G. Mickels-Kokwe Small-scale woodland-based enterprises with outstanding economic potential The case of honey in Zambia G. Mickels-Kokwe National Library of Indonesia Cataloging-in-Publication Data Mickels-Kokwe, G. Small-scale woodland-based enterprises with outstanding economic potential: the case of honey in Zambia/by G. Mickels-Kokwe. Bogor, Indonesia: Center for International Forestry Research (CIFOR), 2006. ISBN 979-24-4673-7 82p. CABI thesaurus: 1. small businesses 2. honey 3. beekeeping 4. commercial beekeeping 5. non- timber forest products 6. production 7. processing 8. trade 9.government policy 10. woodlands 11. case studies 12. Zambia I. Title © 2006 by CIFOR All rights reserved. Published in 2006 Printed by Subur Printing, Jakarta Design and Layout by Catur Wahyu and Eko Prianto Cover photo by Mercy Mwape of the Forestry Department of Zambia Published by Center for International Forestry Research Jl. -

Household Food Security and Nutrition in the Luapula Valley, Zambia
22 Household food security and nutrition in the Luapula Valley, Zambia K. Callens and E.C. Phiri Karel Callens is Chief Technical Adviser and Elizabeth C. Phiri is National Project Coordinator for the Improving Household Food Security and Nutrition Project. he Luapula Valley of northern Zambia has significant Improvements in the production of other important food crops T natural resources, and the main road cutting through the were neglected. valley has attracted many people to the area. Of the In the Luapula Valley, rates of chronic malnutrition and population of 207 000, the majority of people live along the micronutrient deficiencies are unacceptably high. Preliminary lake, the lagoon and the river (Figure 1). Fishing is the results from two nutrition surveys carried out in the area in principal economic activity, and agriculture is practised September 1997 and May 1998 indicated that about 65 percent further inland. The diet of most households is based on two of children under five years of age were stunted because of main staples: cassava and maize. As relish people consume fish chronic protein-energy malnutrition, while 3.7 percent were in those areas with access to water resources and a limited wasted during the rainy season and 2.5 percent during the dry number of less abundant and seasonally available food crops season as a result of acute malnutrition. According to a such as sweet potatoes, groundnuts, bambara nuts, cowpeas participatory rural appraisal carried out by the Zambian and beans as well as indigenous and other vegetables. Palm oil Ministry of Agriculture, Food and Fisheries, the Ministry of is also consumed in a few villages where oil-palm trees are Health, the Ministry of Community Development and Social found. -

Zambia USADF Country Portfolio
Zambia USADF Country Portfolio Overview: Country program established in 1984 and reopened in U.S. African Development Foundation Partner Organization: Keepers Zambia 2004. USADF currently manages a portfolio of 23 projects and one Country Program Coordinator: Guy Kahokola Foundation (KZF) Cooperative Agreement. Total active commitment is $2.9 million. Suite 103 Foxdale Court Office Park Program Manager: Victor Makasa Agricultural investments total $2.6 million. Youth-led enterprise 609 Zambezi Road, Roma Tel: +260 211 293333 investments total $20,000. Lusaka, Zambia Email: [email protected] Email: [email protected] Country Strategy: The program focuses on support to agricultural enterprises, including organic farming as Zambia has been identified as a Feed the Future country. In addition, there are investments in off-grid energy and youth led-enterprises. Enterprise Duration Grant Size Description Mongu Dairy Cooperative Society 2012-2017 $152,381 Sector: Agriculture (Dairy) Limited Town/City: Mongu District in the Western Province 2705-ZMB Summary: The project funds will be used to increase the production and sales of milk through the purchase of improved breed cows, transportation, and storage equipment. Chibusa Home Based Care 2013-2018 $187,789 Sector: Agriculture (Food Processing) Association Town/City: Mungwi District in the Northern Province of Zambia 2925-ZMB Summary: The project funds will be used to provide working capital for purchasing grains, increase milling capacity, build a storage warehouse, and provide funds to improve marketing. Ushaa Area Farmers Association 2013-2018 $94,960 Sector: Agriculture (Rice) Limited Town/City: Mongu District in the Western Province of Zambia 2937-ZMB Summary: The project funds will be used to provide working capital for purchasing rice, build a storage warehouse, and provide funds to improve marketing. -

The Political Ecology of a Small-Scale Fishery, Mweru-Luapula, Zambia
Managing inequality: the political ecology of a small-scale fishery, Mweru-Luapula, Zambia Bram Verelst1 University of Ghent, Belgium 1. Introduction Many scholars assume that most small-scale inland fishery communities represent the poorest sections of rural societies (Béné 2003). This claim is often argued through what Béné calls the "old paradigm" on poverty in inland fisheries: poverty is associated with natural factors including the ecological effects of high catch rates and exploitation levels. The view of inland fishing communities as the "poorest of the poorest" does not imply directly that fishing automatically lead to poverty, but it is linked to the nature of many inland fishing areas as a common-pool resources (CPRs) (Gordon 2005). According to this paradigm, a common and open-access property resource is incapable of sustaining increasing exploitation levels caused by horizontal effects (e.g. population pressure) and vertical intensification (e.g. technological improvement) (Brox 1990 in Jul-Larsen et al. 2003; Kapasa, Malasha and Wilson 2005). The gradual exhaustion of fisheries due to "Malthusian" overfishing was identified by H. Scott Gordon (1954) and called the "tragedy of the commons" by Hardin (1968). This influential model explains that whenever individuals use a resource in common – without any form of regulation or restriction – this will inevitably lead to its environmental degradation. This link is exemplified by the prisoner's dilemma game where individual actors, by rationally following their self-interest, will eventually deplete a shared resource, which is ultimately against the interest of each actor involved (Haller and Merten 2008; Ostrom 1990). Summarized, the model argues that the open-access nature of a fisheries resource will unavoidably lead to its overexploitation (Kraan 2011). -

Full Text Document (Pdf)
Kent Academic Repository Full text document (pdf) Citation for published version Macola, Giacomo (2006) “It Means as If We Are Excluded from the Good Freedom”: Thwarted Expectations of Independence in the Luapula Province of Zambia, 1964-1967. Journal of African History, 47 (1). pp. 43-56. ISSN 0021-8537. DOI https://doi.org/10.1017/S0021853705000848 Link to record in KAR https://kar.kent.ac.uk/7559/ Document Version UNSPECIFIED Copyright & reuse Content in the Kent Academic Repository is made available for research purposes. Unless otherwise stated all content is protected by copyright and in the absence of an open licence (eg Creative Commons), permissions for further reuse of content should be sought from the publisher, author or other copyright holder. Versions of research The version in the Kent Academic Repository may differ from the final published version. Users are advised to check http://kar.kent.ac.uk for the status of the paper. Users should always cite the published version of record. Enquiries For any further enquiries regarding the licence status of this document, please contact: [email protected] If you believe this document infringes copyright then please contact the KAR admin team with the take-down information provided at http://kar.kent.ac.uk/contact.html ‘IT MEANS AS IF WE ARE EXCLUDED FROM THE GOOD FREEDOM’: THWARTED EXPECTATIONS OF INDEPENDENCE IN THE LUAPULA PROVINCE OF ZAMBIA, 1964-1966* BY GIACOMO MACOLA Centre of African Studies, University of Cambridge ABSTRACT: Based on a close reading of new archival material, this article makes a case for the adoption of an empirical, ‘sub-systemic’ approach to the study of nationalist and post- colonial politics in Zambia. -

FORM #3 Grants Solicitation and Management Quarterly
FORM #3 Grants Solicitation and Management Quarterly Progress Report Grantee Name: Maternal and Child Survival Program Grant Number: # AID-OAA-A-14-00028 Primary contact person regarding this report: Mira Thompson ([email protected]) Reporting for the quarter Period: Year 3, Quarter 1 (October –December 2018) 1. Briefly describe any significant highlights/accomplishments that took place during this reporting period. Please limit your comments to a maximum of 4 to 6 sentences. During this reporting period, MCSP Zambia: Supported MOH to conduct a data quality assessment to identify and address data quality gaps that some districts have been recording due to inability to correctly interpret data elements in HMIS tools. Some districts lacked the revised registers as well. Collected data on Phase 2 of the TA study looking at the acceptability, level of influence, and results of MCSP’s TA model that supports the G2G granting mechanism. Data collection included interviews with 53 MOH staff from 4 provinces, 20 districts and 20 health facilities. Supported 16 districts in mentorship and service quality assessment (SQA) to support planning and decision-making. In the period under review, MCSP established that multidisciplinary mentorship teams in 10 districts in Luapula Province were functional. Continued with the eIMCI/EPI course orientation in all Provinces. By the end of the quarter under review, in Muchinga 26 HCWs had completed the course, increasing the number of HCWs who improved EPI knowledge and can manage children using IMNCI Guidelines. In Southern Province, 19 mentors from 4 districts were oriented through the electronic EPI/IMNCI interactive learning and had the software installed on their computers. -

Quarterly Progress Report (January – March, 2017)
Quarterly Progress Report (January – March, 2017) Approval Date: September 30, 2015 QPR Number: [002] Contract/Agreement Number: [AID-611-C-15-00002] Activity Start Date and End Date: [October 1, 2015 to September 30, 2020] Total Award Amount: [$24,389,716.00] Submitted by: [PATH Zambia Office, Stand 11059, Brentwood Lane, Lusaka] [Tel: +260211378950] DISCLAIMER: The author’s views expressed in this report do not necessarily reflect the views of the United States Agency for International Development (USAID), United States President’s Malaria Initiative (PMI) or the United States Government List of Abbreviations ANC Antenatal care BRITE Broad Reach Institute for Training & Education CBO Community-based organizations CD Continuous distribution CHA Community Health Assistant CHAZ Churches Health Association of Zambia CHW Community Health Worker COP Chief of Party CSO Civil Society Organization DHD District Health Director DHO District Health Office DHIS2 District Health Information System 2 DHS Demographic Health Survey DIM District Integrated Meeting EPI Expanded Program on Immunization GRZ Government of the Republic of Zambia GUC Grants under contract HMIS Health Management Information System iCCM Integrated community case management IEC Information, education, and communication IPTp Intermittent preventive treatment in pregnancy IRS Indoor residual spraying ITN Insecticide-treated net JHUCCP Johns Hopkins University Center for Communication Programs LLIN Long Lasting Insecticide Treated Net MACEPA Malaria Control and Elimination Partnership -

Evaluation of Improving Household Food Security and Nutrition in The
Evaluation of Improving Household Food Security and Nutrition in the Luapula Valley Project (IHFSAN) Report of the Evaluation Mission November 2001 Preface The following draft report represents the views of the independent evaluation mission on the performance and achievements of the IHFSAN (Improving Household Food Security and Nutrition in the Luapula Valley) project. The evaluation was initiated with a view to providing the donor, collaborating agencies and FAO with an independent and objective assessment of the current status of implementation of the project, including a review of problems faced. The mission started on 16 September 2001 and ended on 5 October 2001; nine communities involved in the IHFSAN project were visited and numerous collaborators at field, district and provincial level were met. In addition, the mission visited relevant offices in Lusaka. The evaluation was a challenging task given the complex history of the project, the amount of reports, correspondence etc. produced, as well as the relative scarcity of information available on the project’s outcomes and impact. The evaluation mission is most appreciative of the efforts made by the staff and management of IHFSAN, the GRZ officers in the districts visited, and the FAO representation, as well as a range of other individuals who provided information and discussed issues in a frank and constructive manner. The community groups met provided valuable insights and always gave a warm welcome to the mission. In the districts, brainstorming sessions were organized with district officers and Community Support Staff (CSS), which produced sometimes surprising results. The draft report has also benefited from briefings and discussions with ESNP (nutrition), AGPC (crop production) and AGSM (marketing and rural finance) staff members at FAO headquarters in Rome. -

2000 Census of Population and Housing
2000 Census of Population and Housing Published by Central Statistical Office, P. O. Box 31908, Lusaka, Luapula. Tel: 260-01-251377/253468 Fax: 260-01-253468 E-mail: [email protected] Website: www.zamstats.gov.zm September, 2004 COPYRIGHT RESERVED Extracts may be published if Sources are duly acknowledged. Table of Content and Executive Summary i Table of Content and Executive Summary ii Preface The 2000 Census of Population and Housing was undertaken from 16th October to 15th November, 2000. This was the fourth census since Independence in 1964. The other three were carried out in 1969, 1980 and 1990. The 2000 Census operations were undertaken with the use of Grade 11 pupils as enumerators, Primary School Teachers as supervisors, Professionals from within Central Statistical Office and other government departments being as Trainers and Management Staff. Professionals and Technical Staff of the Central Statistical Office were assigned more technical and professional tasks. This report presents detailed analysis of issues on evaluation of coverage and content errors; population, size, growth and composition; ethnicity and languages; economic and education characteristics; fertility; mortality and disability. The success of the Census accrues to the dedicated support and involvement of a large number of institutions and individuals. My sincere thanks go to Co-operating partners namely the British Government, the Japanese Government, the United States Agency for International Development (USAID), United Nations Population Fund (UNFPA), the Norwegian Government, the Dutch Government, the Finnish Government, the Danish Government, the German Government, University of Michigan, the United Nations High Commission for Refugees (UNHCR) and the Canadian Government for providing financial, material and technical assistance which enabled the Central Statistical Office carry out the Census.