Bulgarian National Conference
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
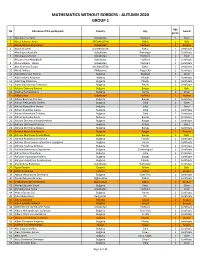
Autumn 2020 Group 1
MATHEMATICS WITHOUT BORDERS - AUTUMN 2020 GROUP 1 Age № Full name of the participant Country City Award group 1 Abdukodirov Tamir Uzbekistan Tashkent 1 Silver 2 Abdul Rahman Amiri AFGHANISTAN KABUL 1 Gold 3 Abdulkadyrov Abdukadyr Uzbekistan Tashkent 1 Bronze 4 Abdullah Amiri AFGHANISTAN KABUL 1 Certificate 5 Abdullayev Asadullo Uzbekistan Namangan 1 Certificate 6 Abdullayev Mirolim Uzbekistan Tashkent 1 Silver 7 Abdumalikov Abdukhalil Uzbekistan Tashkent 1 Certificate 8 Abdurashidov Harun Uzbekistan Tashkent 1 Certificate 9 Abdurrahman Sudays AFGHANISTAN KABUL 1 Certificate 10 Abriol, Willen Philippines Naga City 1 Certificate 11 Adel Dzhyuneyt Topchu Bulgaria Razgrad 1 Silver 12 Adel Emilova Antonova Bulgaria Plovdiv 1 Certificate 13 Adel Oleg Dzhorova Bulgaria Plovdiv 1 Certificate 14 Adela Vladislavova Todorova Bulgaria Pleven 1 Certificate 15 Adelina Dobreva Peneva Bulgaria Burgas 1 Gold 16 Adelina Rumanetsova Bulgaria Varna 1 Silver 17 Adilov Umar Uzbekistan Tashkent 1 Bronze 18 Adison Biserova Tomova Bulgaria Burgas 1 Certificate 19 Adrian Aleksandrov Vasilev Bulgaria Sofia 1 Silver 20 Adrian Alexandrov Ivanov Bulgaria Sofia 1 Silver 21 Adrian Anatoliev Ivanov Bulgaria Sofia 1 Certificate 22 Adrian Momchilov Taslakov Bulgaria Sofia 1 Certificate 23 Adrian Svetoslav Rusev Bulgaria Burgas 1 Certificate 24 Adriana Dimitrova Hadzhinesheva Bulgaria Burgas 1 Certificate 25 Adriana Dimitrova Petrova Bulgaria Vratsa 1 Silver 26 Adriana Dimitrova Zlateva Bulgaria Burgas 1 Certificate 27 Adriana Ilkova Sherbetova Bulgaria Burgas 1 Bronze -

Bulgarian-Swiss Chamber of Commerce
web: www.bscc.bg, e-mail: [email protected] BSCC BULGARIAN-SWISS 2020 CHAMBER OF COMMERCE PROMOTING PARTNERSHIPS ORGANISATION PROFILE March 2020 NMENT IRO ENV Content: TY Bulgarian-Swiss Chamber of Commerce, AddressBILI 2 AINA ST Address by the Swiss Ambassador to BulgariaSU 3 Address by the Bulgarian Ambassador to Switzerland 4 About BSCC 6-7 BSCC in Support of the Business 8 NG BSCC Events 10-11 TI UL NS Bilateral Activities in 2019 12-13 CO BSCC Partners in Bulgaria 14 TH OW BSCC Partners in Switzerland 15 N GR ATIO IC UC OM BSCC – Partner in Social Projects 16 ED ON EC Bulgaria Facts and Figures 17-19 N CS ENTATIO Success Stories of Swiss Investments in Bulgaria 20-27 TI ES GIS EPR Bulgarian-Swiss Chamber of Commerce, Members 28 LO R ES CY RI LI ST DU L PO SOCIA MEM IN P HI CE VI RS NMENT TNE L AD R IRO PA NCIA ENV NA FI Y RG N ENE EA CL NMENT IRO ENV TY BILI AINA ST SU NG TI UL NS CO TH OW N GR ATIO IC UC OM ED ON EC N CS ENTATIO TI ES GIS LO REPR ES CY RI LI ST DU L PO SOCIA MEM IN BSCC BULGARIAN-SWISSP CHAMBER HI CE VI RS NMENT TNE L AD R IRO 2020 PA NCIA ENV OF COMMERCE NA FI Y Dear Reader, RG going backwards and it looks like all stakeholders N ENE EA CL need to adapt to this new reality. Welcome to the 2020 edition of the Bulgarian- Swiss Chamber of Commerce’ brochure. -

Web: E-Mail: [email protected]
web: www.bscc.bg, e-mail: [email protected] BSCC BULGARIAN-SWISS 2020 CHAMBER OF COMMERCE PROMOTING PARTNERSHIPS ORGANISATION PROFILE March 2020 NMENT IRO ENV Content: TY Bulgarian-Swiss Chamber of Commerce, AddressBILI 2 AINA ST Address by the Swiss Ambassador to BulgariaSU 3 Address by the Bulgarian Ambassador to Switzerland 4 About BSCC 6-7 BSCC in Support of the Business 8 NG BSCC Events 10-11 TI UL NS Bilateral Activities in 2019 12-13 CO BSCC Partners in Bulgaria 14 TH OW BSCC Partners in Switzerland 15 N GR ATIO IC UC OM BSCC – Partner in Social Projects 16 ED ON EC Bulgaria Facts and Figures 17-19 N CS ENTATIO Success Stories of Swiss Investments in Bulgaria 20-27 TI ES GIS EPR Bulgarian-Swiss Chamber of Commerce, Members 28 LO R ES CY RI LI ST DU L PO SOCIA MEM IN P HI CE VI RS NMENT TNE L AD R IRO PA NCIA ENV NA FI Y RG N ENE EA CL NMENT IRO ENV TY BILI AINA ST SU NG TI UL NS CO TH OW N GR ATIO IC UC OM ED ON EC N CS ENTATIO TI ES GIS LO REPR ES CY RI LI ST DU L PO SOCIA MEM IN BSCC BULGARIAN-SWISSP CHAMBER HI CE VI RS NMENT TNE L AD R IRO 2020 PA NCIA ENV OF COMMERCE NA FI Y Dear Reader, RG going backwards and it looks like all stakeholders N ENE EA CL need to adapt to this new reality. Welcome to the 2020 edition of the Bulgarian- Swiss Chamber of Commerce’ brochure. -
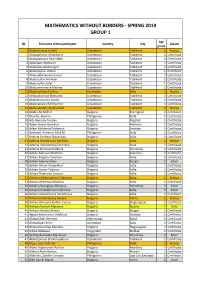
Mathematics Without Borders - Spring 2019 Group 1
MATHEMATICS WITHOUT BORDERS - SPRING 2019 GROUP 1 Age № Full name of the participant Country City Award group 1 Abduazimova Jasmin Uzbekistan Tashkent 1 Bronze 2 Abdugafforov Abdulboriy Uzbekistan Tashkent 1 Certificate 3 Abdujabborov Rashidbek Uzbekistan Tashkent 1 Certificate 4 Abdullaev Abdulaziz Uzbekistan Tashkent 1 Certificate 5 Abdullaev Abdulmajid Uzbekistan Tashkent 1 Certificate 6 Abdullayeva Amina Uzbekistan Tashkent 1 Certificate 7 Abdurakhmanova Zarina Uzbekistan Tashkent 1 Certificate 8 Abduraufov Amirhon Uzbekistan Tashkent 1 Certificate 9 Abduraufov Zafar Uzbekistan Tashkent 1 Certificate 10 Abduraxmonova Madina Uzbekistan Tashkent 1 Certificate 11 Abdurrahmanli Zahra Azerbaijan Baku 1 Bronze 12 Abdusattorova Shahzoda Uzbekistan Tashkent 1 Certificate 13 Abdushukurova Oysha Uzbekistan Tashkent 1 Certificate 14 Abduvahidov Bahtiyornur Uzbekistan Tashkent 1 Certificate 15 Abduvahobov Abduvohob Uzbekistan Tashkent 1 Bronze 16 Abidin Ali Nedret Bulgaria Svilengrad 1 Certificate 17 Abordo, Keanne Philippines Iloilo 1 Certificate 18 Ada Remziev Fevziev Bulgaria Razgrad 1 Certificate 19 Adam Ivanov Goryalov Bulgaria Markovo 1 Certificate 20 Adam Mohamed Mahmud Bulgaria Smolyan 1 Certificate 21 Adelante, Ashanee Misk M Philippines Iloilo 1 Certificate 22 Adelina Encheva Stoyanova Bulgaria Sofia 1 Certificate 23 Adelina Stanimirova Docheva Bulgaria Sofia 1 Bronze 24 Adelina Vladislavova Drumeva Bulgaria Ruse 1 Certificate 25 Adelina Zhivkova Petkova Bulgaria Parvomay 1 Certificate 26 Adrian Adrianov Bozhilov Bulgaria Samokov 1 -
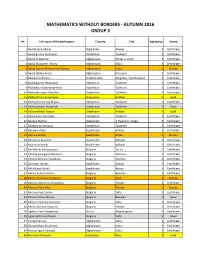
Mathematics Without Borders - Autumn 2016 Group 3
MATHEMATICS WITHOUT BORDERS - AUTUMN 2016 GROUP 3 № Full name of the participant Country City Age group Award 1 Abdrahman Alisher Kazakhstan Almaty 3 Certificate 2 Abdukarimov Samandar Uzbekistan Tashkent 3 Certificate 3 Abdul Al Rahman Afghanistan Mezar-e Sharif 3 Certificate 4 Abdul Muqtader Omary Afghanistan Kabul 3 Certificate 5 Abdul Samad Mohammad Qadeer Afghanistan Kabul 3 Bronze 6 Abdul Wahid Ansari Afghanistan Kandahar 3 Certificate 7 Abdulemir Atayev Turkmenistan Ashgabat, Turkmenistan 3 Certificate 8 Abdullajonov Abdullajon Uzbekistan Tashkent 3 Certificate 9 Abdullayev Saidmuhammad Uzbekistan Tashkent 3 Certificate 10 Abdumannopov Abdullox Uzbekistan Tashkent 3 Certificate 11 Abdurahman Arstanaliev Kyrgyzstan Bishkek 3 Gold 12 Abdurahmonnova Malika Uzbekistan Tashkent 3 Certificate 13 Abdurashidov Abdukodir Uzbekistan Tashkent 3 Silver 14 Abdurashidov Doston Uzbekistan Andijan 3 Gold 15 Abduvalliev Amirzoda Uzbekistan Tashkent 3 Certificate 16 Abekov Mansur Kazakhstan T. Ryskulov village 3 Certificate 17 Abidjanova Somayya Uzbekistan Tashkent 3 Certificate 18 Abilakim Altair Kazakhstan Almaty 3 Certificate 19 Abuova Aizhan Kazakhstan Aktau 3 Bronze 20 Abzalkyzy Ayaulym Kazakhstan Balhash 3 Certificate 21 Abzhanov Erasyl Kazakhstan Balhash 3 Certificate 22 Adel Bahas Karagyozyan Bulgaria Varna 3 Certificate 23 Adelina Georgieva Nikolova Bulgaria Samokov 3 Certificate 24 Adelina Milkova Tsvetkova Bulgaria Shumen 3 Certificate 25 Adilkadyr Nurtas Kazakhstan Almaty 3 Certificate 26 Adilzhanov Nurali Kazakhstan Atyrau 3 Certificate -
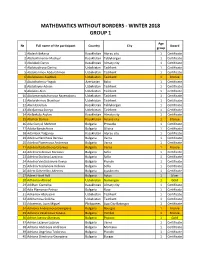
Mathematics Without Borders - Winter 2018 Group 1
MATHEMATICS WITHOUT BORDERS - WINTER 2018 GROUP 1 Age № Full name of the participant Country City Award group 1 Abdesh Bekarys Kazakhstan Atyrau city 1 Certificate 2 Abdrakhmanov Madiyar Kazakhstan Taldykorgan 1 Certificate 3 Abdubek Daryn Kazakhstan Almaty city 1 Certificate 4 Abdukadirova Darina Uzbekistan Tashkent 1 Certificate 5 Abdukarimov Abdurahmon Uzbekistan Tashkent 1 Certificate 6 Abdulazizov Asadbek Uzbekistan Tashkent 1 Bronze 7 Abdulhalimov Yagub Azerbaijan Baku 1 Certificate 8 Abdullayev Adnan Uzbekistan Tashkent 1 Certificate 9 Abdulov Alan Uzbekistan Tashkent 1 Certificate 10 Abdumannobzhonova Rayenabonu Uzbekistan Tashkent 1 Certificate 11 Abdurahimov Shaxriyor Uzbekistan Tashkent 1 Certificate 12 Aben Ersultan Kazakhstan Taldykorgan 1 Certificate 13 Abidjanova Sureya Uzbekistan Tashkent 1 Certificate 14 Abilbekuly Asylan Kazakhstan Almaty city 1 Certificate 15 Abylkali Daniya Kazakhstan Astana city 1 Bronze 16 Ada Gunyul Mehmet Bulgaria Provadia 1 Certificate 17 Adalia Barutchieva Bulgaria Silistra 1 Certificate 18 Adambek Tolganay Kazakhstan Atyrau city 1 Certificate 19 Adelina Nencheva Berova Bulgaria Varna 1 Certificate 20 Adelina Plamenova Andreeva Bulgaria Varna 1 Certificate 21 Adelina Radostinova Grozeva Bulgaria Varna 1 Bronze 22 Adelina Sabinova Borisova Bulgaria Sofia 1 Certificate 23 Adelina Stoilova Lazarova Bulgaria Sofia 1 Certificate 24 Adelina Ventsislavova Koeva Bulgaria Plovdiv 1 Certificate 25 Adelina Yordanova Velkova Bulgaria Sofia 1 Certificate 26 Adem Dzhemilov Ademov Bulgaria Lyaskovets 1 Certificate -

Winter21-Group1
MATHEMATICS WITHOUT BORDERS ‐ WINTER 2021 GROUP 1 Age № Full name of the participant Country City Award group 1 Abboskhudjaev Alisherkhuja Uzbekistan Tashkent 1 Certificate 2 Abdimanap Aisultan Kazakhstan Almaty 1 Certificate 3 Abdrahman Yusufi AFGHANISTAN KABUL 1 Certificate 4 Abdukadirov Abdukadir Uzbekistan Tashkent 1 Certificate 5 Abdukadirov Tamir Uzbekiston Tashkent 1 Certificate 6 Abdukadirova Sahiya Uzbekistan Tashkent 1 Certificate 7 Abdulmuhtarov Zhavohir Uzbekistan Namangan 1 Silver 8 Abdulwaheb Alim AFGHANISTAN KABUL 1 Certificate 9 Abdumalikov Abdukhalil Uzbekistan Tashkent 1 Certificate 10 Abduvahobov Alisher Uzbekistan Namangan 1 Bronze 11 Abdyrahman Ibragim Kazakhstan Almaty 1 Certificate 12 Abidov Salihbek Uzbekistan Tashkent 1 Certificate 13 Abilseit Yerasyl Kazakhstan Almaty 1 Certificate 14 Abriol, Willen Philippines City of Naga 1 Certificate 15 Abzalova Jamila Uzbekistan Tashkent 1 Certificate 16 Adel Dzhyuneyt Topchu Bulgaria Razgrad 1 Gold 17 Adelia Miglenova Davidova Bulgaria Svishtov 1 Certificate 18 Adelina Dobreva Peneva Bulgaria Burgas 1 Silver 19 Adelina Hristomirova Mutafchieva Bulgaria Ruse 1 Certificate 20 Adelina Iskrenova Zhekova Bulgaria Razgrad 1 Bronze 21 Adelina Milenova Nikolova Bulgaria Bozhurishte 1 Certificate 22 Adelina Rumanetsova Bulgaria Varna 1 Silver 23 Adelina Velianova Dobreva Bulgaria Burgas 1 Certificate 24 Adile Nurdzhan Halil Bulgaria Sofia 1 Certificate 25 Adilov Umar Uzbekistan Tashkent 1 Silver 26 Adrian Aleksandrov Ivanov Bulgaria Sofia 1 Silver 27 Adrian Alexandrov Vassilev Bulgaria -
Mathematics Without Borders - Autumn 2019 Group 2
MATHEMATICS WITHOUT BORDERS - AUTUMN 2019 GROUP 2 Age № Full name of the participant Country City Award group 1 Abanilla, Alaina Cassandra P. Philippines Batangas Province 2 Certificate 2 Abduazimova Jasmina Uzbekistan Tashkent 2 Certificate 3 Abduazizona Robiya Uzbekistan Namangan 2 Certificate 4 Abdulfattah Parhiz Afghanistan Herat 2 Certificate 5 Abdulhakim Nurlanbek Uulu Kyrgyzstan Bishkek 2 Certificate 6 Abdumazhidov Nodirbek Uzbekistan Namangan 2 Certificate 7 Abdurahmonova Madina Uzbekistan Tashkent 2 Certificate 8 Abduraufov Zafar Uzbekistan Tashkent 2 Bronze 9 Abdurrahmanli Zahra Azerbaijan Baku 2 Bronze 10 Abdusattorov Abdullox Uzbekistan Tashkent 2 Certificate 11 Abdusattorova Shakhzoda Uzbekistan Tashkent 2 Certificate 12 Abdushukurova Oysha Uzbekistan Tashkent 2 Certificate 13 Abduvahobov Abduvahob Uzbekistan Tashkent 2 Certificate 14 Abduvakhidov Bakhtiyornur Uzbekistan Tashkent 2 Bronze 15 Abelado, Theo Sebastian A. Philippines Naga City 2 Bronze 16 Abrorova Aziza Uzbekistan Tashkent 2 Certificate 17 Achelia Hasan Ismail Bulgaria Razgrad 2 Certificate 18 Ada Remziev Fevziev Bulgaria Razgrad 2 Certificate 19 Adaliya Kalinova Stoilova Bulgaria Plovdiv 2 Certificate 20 Adam Genadievich Burmistrov Bulgaria Burgas 2 Certificate 21 Adam Ivanov Goryalov Bulgaria Markovo 2 Bronze 22 Adam Mohamed Sabri Mohamed Mahmud Bulgaria Smolyan 2 Certificate 23 Adel Ivaylova Kamenova Bulgaria Kyustendil 2 Certificate 24 Adeli Nikolova Boyadzhieva Bulgaria Plovdiv 2 Certificate 25 Adelina Encheva Stoianova Bulgaria Sofia 2 Certificate 26 Adelina -

Web: YOUR CABLE SOLUTION
web: www.bscc.bg, YOUR CABLE SOLUTION OUR PRODUCTS OUR COMPETENCES High and low pressure injection molding Engineering / Consulting Spiral cables / coiled patch cables Manufacturing Energy Chains assembly Laboratory Control cabinets Trading Signal cables Power cord HOWAG OOD BG-7700 Targovishte www.howag.bg BSCC BULGARIAN-SWISS 2018 CHAMBER OF COMMERCE PROMOTING PARTNERSHIPS ORGANISATION PROFILE March 2018 NMENT IRO ENV Content: TY Bulgarian-Swiss Chamber of Commerce, AddressesBILI 2 AINA ST Address by the Swiss Ambassador to BulgariaSU 3 Address by the Bulgarian Ambassador to Switzerland 4 About BSCC 6 The BSCC projects 8 NG BSCC Events 9 TI UL NS Bilateral Activities in 2017 10-11 CO BSCC Partners in Bulgaria 12 TH OW BSCC Partners in Switzerland 13 N GR ATIO IC UC OM BSCC – Partner in Social Projects 14 ED ON EC Bulgaria Facts and Figures 15 N CS ENTATIO Success stories of Swiss investments in Bulgaria 18 TI ES GIS EPR Bulgarian-Swiss Chamber of Commerce, Members 24 LO R ES CY RI LI ST DU L PO SOCIA MEM IN P HI CE VI RS NMENT TNE L AD R IRO PA NCIA ENV NA FI Y RG N ENE EA CL NMENT IRO ENV TY BILI AINA ST SU NG TI UL NS CO TH OW N GR ATIO IC UC OM ED ON EC N CS ENTATIO TI ES GIS LO REPR ES CY RI LI ST DU L PO SOCIA MEM IN BSCC BULGARIAN-SWISSP HI CE VI RS NMENT TNE L AD R IRO 2018 PA NCIA ENV CHAMBER OF COMMERCE NA FI Y Dear Reader, RG BSCC’ Managing Board and Secretariat look forward N ENE EA CL to a better and peaceful 2018 and will continue Welcome to the new 2018 edition of the Bulgarian- to work hard for the development of the Chamber Swiss Chamber of Commerce (BSCC)’ brochure. -

Web: E-Mail: [email protected]
web: www.bscc.bg, e-mail: [email protected] BSCC BULGARIAN-SWISS 2020 CHAMBER OF COMMERCE PROMOTING PARTNERSHIPS ORGANISATION PROFILE March 2020 NMENT IRO ENV Content: TY Bulgarian-Swiss Chamber of Commerce, AddressBILI 2 AINA ST Address by the Swiss Ambassador to BulgariaSU 3 Address by the Bulgarian Ambassador to Switzerland 4 About BSCC 6-7 BSCC in Support of the Business 8 NG BSCC Events 10-11 TI UL NS Bilateral Activities in 2019 12-13 CO BSCC Partners in Bulgaria 14 TH OW BSCC Partners in Switzerland 15 N GR ATIO IC UC OM BSCC – Partner in Social Projects 16 ED ON EC Bulgaria Facts and Figures 17-19 N CS ENTATIO Success Stories of Swiss Investments in Bulgaria 20-27 TI ES GIS EPR Bulgarian-Swiss Chamber of Commerce, Members 28 LO R ES CY RI LI ST DU L PO SOCIA MEM IN P HI CE VI RS NMENT TNE L AD R IRO PA NCIA ENV NA FI Y RG N ENE EA CL NMENT IRO ENV TY BILI AINA ST SU NG TI UL NS CO TH OW N GR ATIO IC UC OM ED ON EC N CS ENTATIO TI ES GIS LO REPR ES CY RI LI ST DU L PO SOCIA MEM IN BSCC BULGARIAN-SWISSP CHAMBER HI CE VI RS NMENT TNE L AD R IRO 2020 PA NCIA ENV OF COMMERCE NA FI Y Dear Reader, RG going backwards and it looks like all stakeholders N ENE EA CL need to adapt to this new reality. Welcome to the 2020 edition of the Bulgarian- Swiss Chamber of Commerce’ brochure. -

Download Sambo Tutorial
Sambo About the Tutorial Sambo is a Soviet martial art and combat sport which is inspired by Jujutsu, Judo, and other forms of martial arts and is mostly referred as a self-defence art. It is one of the most modern forms of martial arts, though it was introduced in the 1920s. Presently it is recognized as one of the authentic styles of international wrestling. This tutorial will provide a brief description about the sport which will include the method of playing, rules, equipment, and other details. Audience This tutorial is meant for anyone who wants to know and learn about Sambo. It is prepared keeping in mind that the reader is unaware about the basics of the sport. It is a basic guide to help a beginner understand Sambo. Prerequisites Before proceeding with this tutorial, you are required to have a passion for Sambo and an eagerness to acquire knowledge on the same. Copyright & Disclaimer Copyright 2016 by Tutorials Point (I) Pvt. Ltd. All the content and graphics published in this e-book are the property of Tutorials Point (I) Pvt. Ltd. The user of this e-book is prohibited to reuse, retain, copy, distribute, or republish any contents or a part of contents of this e-book in any manner without written consent of the publisher. We strive to update the contents of our website and tutorials as timely and as precisely as possible, however, the contents may contain inaccuracies or errors. Tutorials Point (I) Pvt. Ltd. provides no guarantee regarding the accuracy, timeliness, or completeness of our website or its contents including this tutorial. -

Industry Report Repair of Computers and Personal and Household Goods 2016 BULGARIA
Industry Report Repair of computers and personal and household goods 2016 BULGARIA seenews.com/reports This industry report is part of your subcription access to SeeNews | seenews.com/subscription CONTENTS I. KEY INDICATORS II. INTRODUCTION III. REVENUES IV. EXPENSES V. PROFITABILITY VI. EMPLOYMENT 1 SeeNews Industry Report In 2015 there were a total of 3,589 companies operating in the industry. In 2014 their number totalled 3,546. I. KEY INDICATORS NUMBER OF COMPANIES IN REPAIR OF COMPUTERS AND The Repair of computers and personal and household PERSONAL AND HOUSEHOLD GOODS INDUSTRY BY SECTORS goods industry in Bulgaria was represented by 3,576 SECTOR 2016 2015 2014 companies at the end of 2016, compared to 3,589 in the REPAIR OF OTHER PERSONAL AND 863 857 815 previous year and 3,546 in 2014. HOUSEHOLD GOODS REPAIR OF HOUSEHOLD APPLIANCES AND 856 893 903 The industry's net profit amounted to BGN 16,725,000 in HOME AND GARDEN EQUIPMENT 2016. REPAIR OF COMPUTERS AND PERIPHERAL 739 729 690 EQUIPMENT The industry's total revenue was BGN 141,329,000 in 2016, REPAIR OF CONSUMER ELECTRONICS 334 340 357 up by 8.74% compared to the previous year. REPAIR OF FOOTWEAR AND LEATHER GOODS 230 241 238 REPAIR OF FURNITURE AND HOME 219 198 208 FURNISHINGS The combined costs of the companies in the Repair of REPAIR OF COMMUNICATION EQUIPMENT 179 167 172 computers and personal and household goods industry REPAIR OF WATCHES, CLOCKS AND JEWELLERY 156 164 163 reached BGN 123,347,000 in 2016, up by 12.44% year-on- year.